Blood testing plays a pivotal role in modern medical diagnostics. Statistics show that approximately 70% of clinical decisions rely on laboratory test results, making blood specimen quality directly critical to the reliability of test outcomes. Vacuum blood collection tubes, as the core tool for blood collection, not only affect the accuracy of test data but also directly impact patient diagnosis, treatment efficacy, and safety.
Since vacuum blood collection technology emerged in the 1960s, it has become the standard blood collection method in healthcare facilities worldwide. However, in daily practice, overlooking operational details often leads to hemolysis, clotting, insufficient blood volume, and other issues. These seemingly minor operational errors can cause test result deviations, ultimately affecting clinical diagnostics accuracy.
This guide approaches the subject from a practical perspective, providing detailed analysis of every critical aspect of vacuum blood collection tubes usage, offering healthcare professionals comprehensive and practical operational guidelines to ensure every blood specimen meets optimal quality standards.
Overview of Vacuum Blood Collection Tubes
What Are Vacuum Blood Collection Tubes?
Vacuum blood collection tubes are blood collection devices that operate on negative pressure principles. The system consists of three core components: vacuum collection tubes, disposable collection needles, and needle holders. The working principle utilizes factory pre-established vacuum environments. After the collection needle punctures a blood vessel, the pressure differential between inside and outside the tube (typically -0.6 to -0.8 atmospheres) automatically draws blood, achieving quantitative, sterile blood collection.
Tube walls are typically made from borosilicate glass or PET plastic, providing excellent chemical stability and transparency. The tube opening features specially designed rubber stoppers that maintain internal vacuum while allowing needle penetration. The tube bottom is designed as conical or flat-bottomed, facilitating thorough blood mixing and subsequent processing.

Main Types and Detailed Applications
Red-Top Tubes (Non-Additive Serum Tubes)

Red-top tubes are the most basic type of vacuum collection tube, containing no chemical additives and relying on natural blood coagulation to obtain serum. After collection, fibrinogen in the blood converts to fibrin under thrombin action, forming blood clots and releasing clear serum.
Applicable test items include:
- Chemistry panels: liver function (ALT, AST, ALP, GGT), kidney function (BUN, creatinine), lipid panels (total cholesterol, triglycerides, HDL, LDL), glucose, electrolytes
- Immunology tests: thyroid function, tumor markers, hormone levels, autoantibodies
- Infectious disease markers: hepatitis panel, hepatitis C antibody, HIV antibody, syphilis antibody
Usage considerations: After collection, allow 30-60 minutes at room temperature for complete coagulation, then centrifuge to separate serum. Insufficient coagulation time leads to fibrin precipitation, affecting laboratory test results.
Purple-Top Tubes (EDTA Anticoagulant Tubes)
Purple-top tubes contain ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, typically EDTA-K2 or EDTA-K3. EDTA chelates calcium ions in blood, blocking the coagulation cascade and maintaining blood’s liquid state. Additionally, EDTA preserves blood cell morphology and size, making it ideal for hematologic testing.
Applicable test items include:
- Complete blood count: red blood cell count, white blood cell count, platelet count, hemoglobin, hematocrit
- Blood typing: ABO blood group, Rh system testing
- Crossmatch testing: ensuring transfusion safety
- Blood cell morphology: blood smear microscopy, abnormal cell identification
Key points: After collection, immediately and gently invert 5-8 times to ensure thorough EDTA-blood mixing. Insufficient mixing causes localized clotting, affecting blood specimen quality. Testing should be completed within 6 hours of collection to preserve cell morphology integrity.
Light Blue-Top Tubes (Sodium Citrate Anticoagulant Tubes)

Light blue-top tubes contain 3.2% or 3.8% sodium citrate solution, with the same calcium-chelating anticoagulation principle. Sodium citrate’s distinctive feature is reversible anticoagulation – calcium ion addition during testing can restart the coagulation process. This characteristic makes it the dedicated tube type for coagulation studies.
Applicable test items include:
- Coagulation screening: prothrombin time (PT), activated partial thromboplastin time (aPTT)
- International normalized ratio (INR): monitoring warfarin and other anticoagulant therapy efficacy
- Fibrinogen (FIB): assessing fibrinogen levels
- D-dimer: diagnosing thrombotic diseases
- Coagulation factor activity testing
Critical requirements: Blue-top tubes require extremely strict blood-to-anticoagulant ratios, maintaining a 1:9 ratio (4.5ml blood to 0.5ml sodium citrate). Insufficient or excessive blood volume severely affects coagulation parameter accuracy. After collection, invert 3-4 times and complete testing within 4 hours.
Green-Top Tubes (Heparin Anticoagulant Tubes)
Green-top tubes contain sodium heparin or lithium heparin. Heparin enhances antithrombin III activity, inhibiting thrombin and factor Xa for anticoagulation. Heparin’s advantages include rapid anticoagulation without significantly altering blood osmotic pressure, maintaining natural cellular states.
Applicable test items include:
- Blood gas analysis: pH, oxygen pressure (PO2), carbon dioxide pressure (PCO2), bicarbonate (HCO3-)
- Electrolyte testing: especially rapid sodium, potassium, chloride detection
- Certain emergency chemistry panels: glucose, creatinine, blood urea nitrogen
- Chromosome analysis: maintaining cell viability for cell culture
Precautions: Heparin interferes with certain tests, such as platelet aggregation studies, making it unsuitable for coagulation testing. Mix immediately after collection; blood gas specimens should be tested promptly, preferably within 30 minutes.
Gray-Top Tubes (Sodium Fluoride Anticoagulant Tubes)
Gray-top tubes contain composite additives of sodium fluoride and EDTA. Sodium fluoride acts as a glycolytic inhibitor, preventing red blood cells from continuing to consume glucose in blood, while EDTA provides anticoagulation. This combination ensures blood glucose level stability, making it the dedicated tube type for glucose testing.
Applicable test items:
- Glucose testing: fasting glucose, postprandial glucose, random glucose
- Glucose tolerance testing: oral glucose tolerance test (OGTT)
- Lactate testing: diagnosing certain metabolic diseases
Special requirements: Gray-top tubes are primarily for glucose testing, as sodium fluoride interferes with certain enzyme activities, making them unsuitable for other chemistry tests. Mix immediately after collection; glucose levels remain stable for over 24 hours even at room temperature.
Pre-Use Preparation
Comprehensive Equipment Inspection
Thorough Vacuum Blood Collection Tube Examination
Before using vacuum blood collection tubes, systematic quality checks must be performed as the first line of defense for successful collection and blood specimen quality.
First, check tube wall integrity. Carefully observe for cracks, damage, or scratches on tube walls. Even microscopic cracks can cause vacuum loss, affecting collection effectiveness. For glass tubes, pay special attention to openings and bottoms for chips; for plastic tubes, check for deformation or aging.
Vacuum level inspection is critical. The correct method is gently pressing the stopper center, feeling resistance and rebound. Normal vacuum tubes should show significant resistance when lightly pressed, with stoppers quickly rebounding to original position upon release. If stoppers are too soft or rebound slowly, vacuum is insufficient and use should be discontinued. Additionally, observe whether stoppers are slightly concave inward, which is normal.
Expiration date verification cannot be overlooked. Check production dates and expiration markings on outer packaging or tube walls, ensuring use within validity periods. Expired products may experience anticoagulant failure or vacuum degradation. Also consider storage conditions – if packaging is damaged or storage environments inappropriate, exercise caution even if unexpired.
Label information verification includes checking tube cap colors match required test items, confirming tube specifications (2ml, 5ml, 10ml) suit collection volume needs, and verifying batch numbers are clearly legible for quality traceability.
Professional Selection and Inspection of Collection Needles
Collection needle selection directly affects collection success rates and patient comfort. Needle specifications are typically indicated by gauge numbers – higher numbers indicate thinner needles. Common specifications include: 20-gauge needles (0.9mm outer diameter) for routine adult venipuncture; 21-gauge needles (0.8mm outer diameter) for patients with average vascular conditions; 22-gauge needles (0.7mm outer diameter) for elderly patients or those with thinner vessels; 23-25 gauge needles for pediatric patients or those with poor vascular conditions.
Needle quality inspection points: Check needle sharpness, visually inspecting needle tips for completeness without burrs or bending; verify packaging integrity ensuring sterile packaging is undamaged; check needle-to-needle holder connection compatibility; confirm needle shields are securely installed without looseness.
Functional Testing of Needle Holders
Needle holders are intermediary devices connecting collection needles and vacuum tubes, with performance directly affecting operational convenience and safety.
Inspection points include: spring mechanism testing through multiple tube insertions and removals, ensuring normal spring rebound; threaded connection checks ensuring tight needle connections without looseness; internal cavity cleanliness checks ensuring no blood residue or contaminants; external integrity checks ensuring no cracks or deformation.
Environmental Preparation Importance
Standardized Blood Collection Environment Requirements
Ideal collection environments should feature: sufficient, evenly distributed lighting avoiding shadows that impair vessel identification; appropriate temperature (68-77°F), as cold environments cause vasoconstriction affecting collection; humidity control at 40-60% avoiding excessive dryness or moisture; good air circulation without direct drafts; noise control within reasonable ranges creating quiet environments; cleanliness and hygiene with regular disinfection avoiding cross-infection risks.
Comprehensive Auxiliary Supply Preparation
Disinfection supplies: 70% isopropyl alcohol pads or povidone-iodine pads for skin disinfection; disposable disinfection wipes for equipment surface cleaning; hand sanitizer ensuring operator hand hygiene.
Hemostasis supplies: medical tourniquets selecting appropriate width and elasticity; disposable adhesive tape for cotton ball securing; sterile cotton balls or gauze for compression hemostasis; sterile gauze for special situations.
Safety protection supplies: disposable medical gloves selecting appropriate sizes ensuring operational flexibility; protective eyewear preventing blood splatter; disposable aprons or protective gowns; sharps containers for safe disposal of used collection needles.
Critical Considerations During Operation
Scientific Blood Collection Sequence
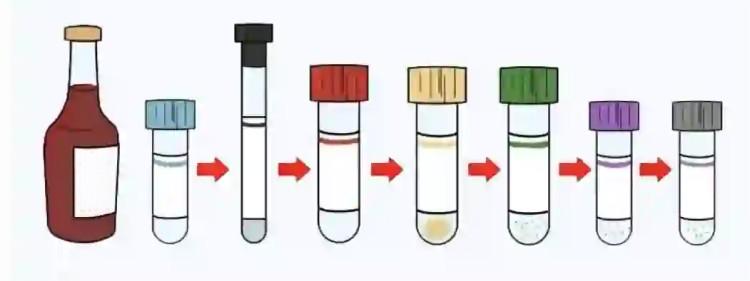
Blood collection sequence establishment is based on cross-contamination prevention principles, as different additive interactions can seriously affect laboratory test results accuracy. The International Federation of Clinical Chemistry (IFCC) and Clinical and Laboratory Standards Institute (CLSI) provide clear recommendations.
Detailed Standard Collection Sequence:
- Blood Culture Bottles (if required) Blood cultures require the highest sterile conditions, necessitating first collection. After successful puncture, first connect blood culture bottles, avoiding any potential contamination. Blood cultures typically require two sets (aerobic and anaerobic bottles), with 8-10ml per bottle for adults, calculated by weight for children.
- Red-Top Tubes (Non-Additive Serum Tubes) Non-additive tubes rank second, avoiding contamination from other tube additives. Containing no chemical substances, even minimal tissue fluid mixing won’t affect most test items. This sequence ensures blood specimen “purity.”
- Light Blue-Top Tubes (Sodium Citrate Coagulation Tubes) Coagulation testing requires high specimen purity, as any trace EDTA or heparin contamination significantly affects clotting times. Therefore, blue-top tubes must be used before other anticoagulant tubes. Particularly note that when collecting only blue-top tubes, first use a small red-top “discard tube” collecting 1-2ml blood to clear tissue fluid and clots potentially mixed during puncture.
- Green-Top Tubes (Heparin Anticoagulant Tubes) Heparin has relatively minimal impact on subsequent EDTA tubes, but sequence control remains necessary. Heparin’s anticoagulation mechanism doesn’t interfere with EDTA chelation, allowing placement before EDTA tubes.
- Purple-Top Tubes (EDTA Anticoagulant Tubes) EDTA is the strongest anticoagulant with persistent chelating action. EDTA contamination of coagulation tubes causes significantly prolonged clotting times, producing false-positive results. Therefore, EDTA tubes must follow coagulation tubes.
- Gray-Top Tubes (Sodium Fluoride Tubes) Sodium fluoride is toxic with cellular cytotoxic effects, necessitating final collection. Additionally, sodium fluoride interferes with multiple enzyme activity tests, requiring contamination prevention of other specimens.
Precise Blood Collection Technique Control
Scientific Venous Selection Principles
Venous selection is key to blood collection success. Primary sites are antecubital fossa superficial veins, including basilic vein, cephalic vein, and median cubital vein. These vessels have relatively fixed positions, appropriate diameters, fewer surrounding nerves, and minimal pain sensation.
Phlebotomy techniques require careful consideration of vessel characteristics. The basilic vein, located medially in the antecubital fossa, is the largest superficial vein with highest collection success rates, but proximity to the brachial artery and median nerve requires careful depth control during puncture. The cephalic vein, located laterally in the antecubital fossa, is relatively safe but sometimes has significant anatomical variations. The median cubital vein connects basilic and cephalic veins, centrally positioned, representing an excellent choice.
If antecubital fossa veins are unsuitable, consider forearm cephalic or basilic veins, or dorsal hand venous networks. However, dorsal hand veins are thinner with slower blood flow, potentially extending collection time while causing greater patient discomfort.
Venous assessment points: palpate vessel elasticity – good vessels should be elastic, rapidly refilling after compression; observe vessel direction, selecting straight segments for puncture; evaluate vessel depth – excessively deep vessels are difficult to puncture, while superficial vessels are easily penetrated; examine skin conditions, avoiding scars, infections, or edematous areas.
Technical Points of Tourniquet Use
Proper tourniquet use is crucial for venous engorgement, but improper use creates problems. Standard tourniquet placement should be 2-4 inches above the puncture site, typically in the upper third of the upper arm. Tourniquet tightness should obstruct venous return without affecting arterial flow, with distal arterial pulses palpable as the standard.
Tourniquet time is strictly controlled within 1 minute, preferably under 30 seconds. Prolonged tourniquet application causes hemoconcentration, pH reduction, and certain analyte concentration changes. If initial puncture fails requiring re-tourniquet application, first release the tourniquet, allow normal circulation restoration for 2 minutes, then reapply.
Tourniquet method uses slip-knot technique for quick release. After successful venipuncture with blood beginning to flow into collection tubes, immediately release the tourniquet to prevent blood flow obstruction affecting collection speed and blood quality.
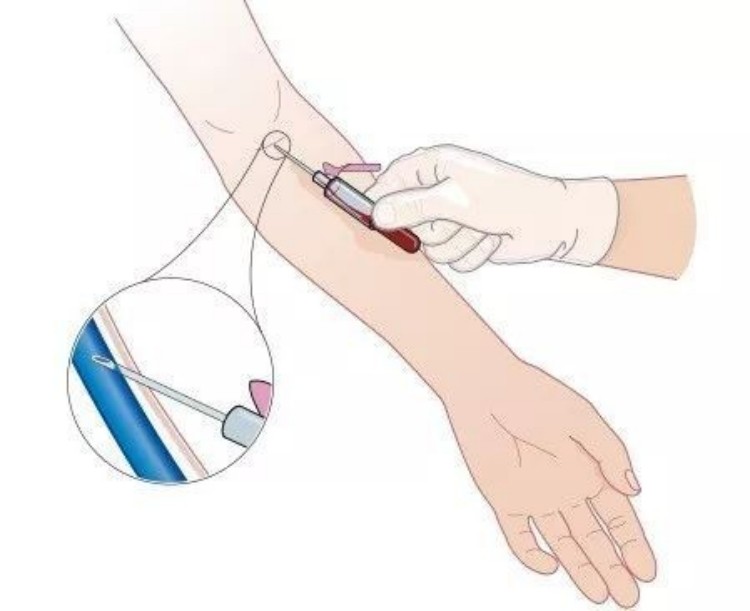
Precise Puncture Technique Control
Pre-puncture skin disinfection uses concentric circle method, expanding outward from puncture site with disinfection diameter of at least 2 inches. Disinfectant contact time should be adequate – alcohol disinfection requires 30+ seconds, povidone-iodine requires 1-2 minutes. After disinfection, allow natural air-drying, avoiding puncture in wet conditions.
Puncture angle control between 15-30 degrees, with specific angles adjusted based on vessel depth. Shallower vessels allow smaller angles; deeper vessels require appropriately larger angles. Puncture with bevel up reduces vascular intimal injury.
Needle insertion technique requires steadiness, accuracy, and speed. “Steady” means stable needle-holding hand avoiding trembling; “accurate” means precisely targeting vessel direction, penetrating vessel walls once; “speed” means decisive insertion movement avoiding hesitation causing patient anxiety.
Post-flashback handling: When collection needles successfully enter veins, small amounts of blood enter the needle’s transparent connecting tube, called “flashback.” At this point, don’t continue advancing; slightly lower puncture angle and stabilize the needle along vessel direction.
Standardized Vacuum Tube Insertion Operations
When inserting vacuum collection tubes into needle holders, ensure collection needles completely penetrate rubber stoppers. Insertion movements should be decisive and firm – hesitation leads to incomplete penetration affecting blood flow. However, avoid excessive force causing needle penetration through tube bottoms.
During blood flow, vacuum blood collection tubes should maintain slight inclination avoiding blood directly impacting tube walls causing hemolysis. Simultaneously observe blood flow speed – under normal conditions, blood should flow continuously and uniformly. If blood flow interrupts or slows, needle position may be inappropriate or vessel spasm may occur, requiring appropriate adjustment.
During multiple tube collection, maintain stable needle position with each tube change, avoiding excessive vessel movement causing vascular injury or puncture failure. Tube changing movements should be gentle and swift, preventing air entry into vessels.
Collection Volume Precision Control
Different Tube Type Volume Requirements
Each vacuum blood collection tube type has specific volume requirements affecting not only test accuracy but also test equipment normal operation.
Light blue-top tubes (coagulation tubes) have the strictest volume requirements. Standard 4.5ml blue-top tubes must be filled to standard graduation lines, ensuring strict 9:1 blood-to-sodium citrate ratios. Insufficient volume causes relatively excessive sodium citrate concentrations, over-anticoagulation, and artificially prolonged clotting times; excessive volume causes relatively insufficient anticoagulant with microclot formation affecting laboratory test results. Clinically common volume errors include: collection volumes under 4ml or over 5ml are unacceptable.
Purple-top tubes (EDTA tubes) have relatively flexible volume requirements but must meet minimum requirements. Generally, 2ml tubes require at least 1.5ml, 5ml tubes require at least 3ml, ensuring adequate testing volumes. Insufficient volume causes relatively excessive EDTA concentrations, potentially causing cellular morphologic changes and platelet aggregation.
Red-top tubes (serum tubes) and green-top tubes (heparin tubes) have relatively lenient volume requirements but must reach minimum volumes satisfying test needs. Typically require collection to 70%+ of capacity.
Consequences of Insufficient Blood Volume
Insufficient collection volume produces multiple adverse consequences:
Test accuracy impacts: anticoagulant-to-blood ratio imbalances cause test result deviations; insufficient blood volume may prevent normal test equipment operation; certain items cannot be tested due to inadequate specimen volumes.
Increased clinical risks: incorrect test results may mislead clinical diagnosis; recollection requirements increase patient suffering; delayed diagnosis and treatment timing.
Quality control issues: affects laboratory quality indicators; increases specimen rejection rates; wastes medical resources.
Post-Collection Processing
Critical Immediate Processing Steps
Scientific Principles and Practice of Mixing Techniques
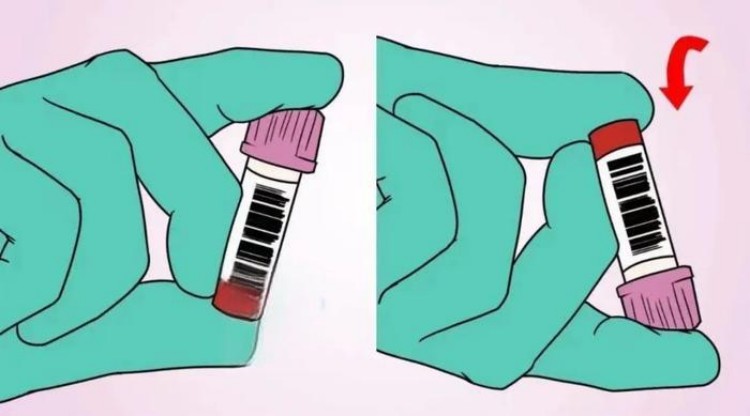
Mixing is the key step ensuring thorough blood-additive combination, with different collection tube types requiring different mixing methods and frequencies.
EDTA tube mixing: requires 5-8 gentle inversions, with each inversion completely turning tubes 180 degrees, allowing blood to flow completely from one end to the other. Mixing movements should be gentle, avoiding vigorous shaking causing red blood cell destruction. Proper mixing should begin within 30 seconds of collection completion, with each inversion taking approximately 1-2 seconds and the entire mixing process controlled within 15-20 seconds. Insufficient mixing manifestations include clot adherence to tube walls, blood layering phenomena, and abnormal blood cell counts.
Sodium citrate tube mixing: requires 3-4 gentle inversions, as sodium citrate’s anticoagulation action is relatively mild, with excessive mixing potentially activating platelets. Mixing movements should likewise be gentle, avoiding foam production.
Heparin tube mixing: requires 5-6 gentle inversions. Heparin anticoagulates rapidly but needs thorough mixing ensuring every blood portion contacts anticoagulant.
Serum tubes typically don’t require immediate mixing and should remain upright allowing natural blood coagulation. Immediate mixing disrupts coagulation processes, affecting serum separation.
Hemolysis Prevention and Identification
Hemolysis is one of the most common blood specimen quality issues, seriously affecting multiple test result accuracy.
Primary hemolysis causes: mechanical injury such as vigorous shaking, rapid syringe pushing/pulling, excessively fine needles, excessive blood flow speed; osmotic injury such as excessive additive concentrations in collection tubes, inappropriate storage temperatures, frozen storage; chemical injury such as disinfectant residue, abnormal pH levels.
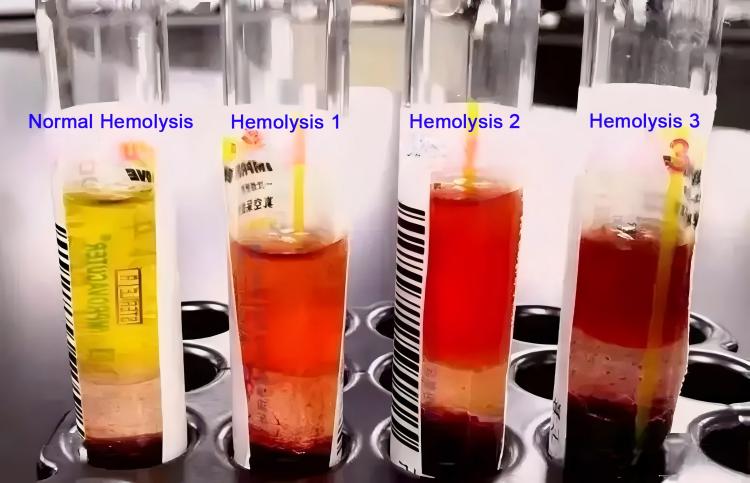
Hemolysis identification methods: visual observation of blood or serum color – mild hemolysis appears light pink, moderate hemolysis appears red, severe hemolysis appears dark red or brownish-red; laboratories can quantitatively assess hemolysis levels by testing hemoglobin concentrations; blood smear observation can reveal fragmented red blood cells and free hemoglobin.
Hemolysis prevention measures: select appropriately sized collection needles avoiding excessively fine needles; control blood flow speed avoiding excessive flow; gentle mixing after collection avoiding vigorous shaking; appropriate storage and transport conditions; timely testing avoiding prolonged standing.
Standardized Specimen Identification Management
Complete Identification Information Requirements
Specimen identification is important for ensuring accurate test result attribution – identification errors can lead to serious medical accidents.
Required identification information includes: patient names using actual patient names avoiding nicknames or substitutes; gender identification important for certain hormone test items; age information as certain test reference ranges correlate with age; unique identification codes such as outpatient numbers, inpatient numbers, or social security numbers; collection date and time precise to hours and minutes; collector signatures for quality traceability and issue queries; specimen types clearly indicating serum, plasma, or whole blood.
Identification Material and Method Selection
Label materials should select waterproof, acid-alkali resistant medical labels ensuring clarity under various storage and transport conditions.
Identification methods: handwritten identification using waterproof ink pens with clear, neat handwriting; barcode printed identification improving efficiency and accuracy; electronic identification systems achieving information management.
Identification timing: optimal timing is immediately after collection completion in front of patients, avoiding confusion from collecting multiple patient specimens then uniform identification later. Identification positions should be on collection tube sidewalls, not covering volume graduations and color identifications.
Standardized Storage and Transport Requirements
Temperature Control Importance
Different test items have different storage temperature requirements – inappropriate temperature control causes test result deviations or even specimen rejection.
Room temperature storage (59-77°F) applicable items: most chemistry panel items such as liver function, kidney function, lipid panels; routine immunology items; coagulation function tests (but requiring testing within 4 hours).
Refrigerated storage (36-46°F) applicable items: certain hormone tests such as insulin, C-peptide; certain tumor markers; blood gas analysis specimens (short-term storage); certain microbiology culture specimens; vitamin testing.
Frozen storage (-4°F and below) special requirements: certain special protein tests; viral nucleic acid testing; long-term preserved research specimens. However, frozen storage destroys cellular structures, making it unsuitable for complete blood count and other cellular examinations.
Storage Container and Environmental Requirements
Specimen storage should use dedicated specimen racks or specimen boxes ensuring collection tubes remain upright, avoiding tipping or rolling. Storage environments require darkness, ventilation, cleanliness, away from heat sources and chemical reagents. Refrigerated storage requires dedicated specimen refrigerators with regular temperature monitoring and recording ensuring temperature stability.
For specimens requiring special protection, such as light-protected bilirubin specimens, use black bags or aluminum foil wrapping; for CO-Hb testing specimens, require sealed preservation avoiding gas exchange.
Transport Process Quality Control
Transport time control: complete blood count specimens should be tested within 6 hours of collection; coagulation function specimens should be tested within 4 hours; blood gas analysis specimens should be tested within 30 minutes; most chemistry items can be tested within 24 hours.
Transport method selection: intra-hospital transport typically uses dedicated transport carts or transport boxes; long-distance transport requires insulated boxes or cold chain transport; special specimens need personal escort.
Transport process considerations: maintain collection tubes upright avoiding inversion or tilting; avoid vigorous shaking and impacts; maintain appropriate temperature environments; maintain transport records including time, temperature, personnel information.
Common Issues and Solutions
In-Depth Analysis of Collection Failures
Insufficient Vacuum Diagnosis and Management
Insufficient vacuum is a common cause of collection failure, manifesting as slow blood flow, insufficient volume, or complete inability to flow.
Diagnostic methods: observe blood flow speed – under normal conditions blood should flow continuously and stably; check stopper rebound – light stopper pressure should show obvious resistance; auscultation method – placing collection tubes near ears, normal vacuum tubes should produce slight “hissing” sounds when needles are inserted.
Common cause analysis: expired collection tubes or inappropriate storage causing natural vacuum degradation; microscopic tube wall cracks causing air leaks; rubber stopper aging losing sealing properties; transport process impacts causing vacuum loss; temperature changes causing thermal expansion and contraction effects.
Prevention measures: establish strict inventory management systems executing first-in-first-out principles; regularly inspect inventory collection tube quality status; improve storage environments avoiding extreme temperatures and humidity; provide transport process protection avoiding vigorous shaking.
Emergency management: immediately replace new collection tubes; if multiple consecutive collection tubes show problems, check for batch quality issues; record problem collection tube batch numbers and manufacturer information for quality traceability.
Systematic Solutions for Vascular Puncture Technical Issues
Inappropriate puncture techniques are another important cause of collection failure, requiring systematic improvement from technical perspectives.
Puncture angle problems: excessive angles easily penetrate vessels causing hematomas; insufficient angles may fail to penetrate vessel walls or only penetrate one side. Solutions involve adjusting angles based on vessel depth – general vessel puncture angles controlled at 15-30 degrees, specifically based on vessel positions.
Puncture depth control: excessive depth may injure posterior vessel walls or surrounding tissues; insufficient depth may fail to completely enter vessel lumens. Proper depth control requires judgment based on vessel thickness and depth – general insertion depths are 1/3-1/2 of vessel diameters.
Puncture site selection: avoid vessel bifurcations and valve locations; select straighter vessel portions; avoid scar and sclerotic areas. If initial punctures fail, reselected puncture sites should be at least 2cm from original puncture sites.
Vessel stabilization techniques: use non-needle-holding hand thumbs to pull skin downward 2-3cm below puncture sites, stabilizing vessels preventing sliding; for elderly patients with loose skin and high vessel mobility, stabilization is especially important.
Patient Factor-Induced Collection Difficulties
Dehydrated patient management: dehydration causes insufficient blood volume, poor venous filling, vessel collapse. Management methods include appropriate fluid supplementation improving blood volume; selecting larger veins; extending tourniquet time but not exceeding 2 minutes; having patients make fists promoting venous engorgement.
Vascular sclerosis patients: common in elderly and diabetic patients with vessel wall sclerosis and poor elasticity. Technical points include selecting relatively softer vessel segments; appropriately increasing puncture angles; decisive needle insertion avoiding hesitation; considering shallower dorsal hand veins.
Obese patient special management: thick subcutaneous fat makes vessel localization difficult. Solution strategies include thorough palpation with flashlight transillumination assistance if necessary; selecting larger veins; appropriately increasing puncture angles and depths; considering ultrasound-assisted localization.
Specimen Quality Issue Prevention and Management
In-Depth Hemolysis Analysis
Hemolysis is graded as mild, moderate, and severe, with different degrees affecting various test items differently.
Mild hemolysis (hemoglobin <0.5g/L): minimal impact on most chemistry items such as glucose, blood urea nitrogen, creatinine; slight impact on potassium ions and LDH but usually within acceptable ranges; complete blood count testing basically unaffected.
Moderate hemolysis (hemoglobin 0.5-2.0g/L): significantly affects potassium ion, LDH, AST test results; certain impact on bilirubin, total protein items; complete blood count hemoglobin measurements are interfered.
Severe hemolysis (hemoglobin >2.0g/L): seriously affects most chemistry indicator accuracy; serum appears obviously red, preventing most testing; requires recollection.
Laboratory hemolysis assessment: visual method observing serum color changes; spectrophotometric method measuring serum hemoglobin content; automated testing systems typically have hemolysis index testing functions.
Systematic hemolysis prevention measures: technical aspects standardizing collection operations avoiding mechanical injury; equipment aspects selecting appropriate collection needle specifications, regularly calibrating centrifuge speeds; environmental aspects controlling storage and transport temperatures; management aspects strengthening personnel training, establishing quality monitoring systems.
Coagulation Issue Identification and Management
Partial coagulation is a common quality issue in anticoagulant tubes, with primary causes including insufficient mixing, inadequate collection volume, and anticoagulant failure.
Early identification methods: visual observation of blood fluidity – normal anticoagulated blood should flow freely; gentle tube shaking observing for clots; microscopic observation for fibrin threads or platelet aggregation.
Coagulation degree assessment: mild coagulation manifesting as few fibrin threads with minimal complete blood count impact; partial coagulation manifesting as obvious clots seriously affecting blood cell counts; complete coagulation manifesting as completely solidified blood preventing testing.
Specific prevention measure implementation: begin mixing within 30 seconds of collection completion; ensure collection volumes meet standard requirements; check anticoagulant expiration or deterioration; train operators in proper mixing techniques.
Lipemic Interference Management Strategies
Lipemia refers to blood containing large amounts of lipid particles, making serum appear milky white or turbid, primarily affecting spectrophotometric testing.
Lipemia grading: mild lipemia with slightly turbid serum affecting few items; moderate lipemia with obviously turbid serum affecting most chemistry items; severe lipemia with milky white serum seriously interfering with testing.
Technical management methods: high-speed centrifugation extending centrifuge time or increasing speed; dilution method using saline for specimen dilution; ultrasonic treatment destroying lipid particles; lipid removal reagent treatment.
Clinical management strategies: recommend 12-hour fasting before patient collection; provide dietary guidance for diabetic and hyperlipidemic patients; note lipemic interference in emergency patient reports; arrange patient follow-up if necessary.
Special Situation Management
Specialized Collection Techniques for Special Populations
Professional Pediatric Patient Management
Pediatric blood collection is technically challenging, requiring consideration of physiological, psychological, and technical factors.
Physiological characteristic considerations: pediatric vessels are small and thin-walled, easily penetrated; relatively small blood volumes preventing excessive collection; fast metabolism with rapid blood circulation; relatively large surface areas with easy heat loss potentially causing temperature drops.
Psychological factor management: most children fear blood collection requiring parental cooperation for comfort; distraction methods such as music, storytelling; operations should be swift and accurate reducing suffering time; appropriate post-collection rewards such as stickers, small toys.
Detailed technical points: select 23-25 gauge fine needles reducing trauma; calculate collection volumes by weight, generally not exceeding 1% of body weight; prioritize scalp veins or dorsal hand veins avoiding active joints; consider topical anesthetic cream for pain reduction; operate gently avoiding excessive force.
Special infant considerations: newborns can use heel stick methods; infants under 6 months prioritize femoral or external jugular veins; require dedicated personnel for infant restraint ensuring safety; closely monitor post-collection preventing excessive bleeding.
Detailed Elderly Patient Management
Elderly patients require special technical considerations and humanitarian care due to physiological function decline.
Physiological change impacts: decreased vascular elasticity with easy rupture and bleeding; loose skin with high vessel mobility making puncture localization difficult; potentially abnormal coagulation function with prolonged hemostasis time; possible multiple disease complications such as diabetes, hypertension.
Vascular selection strategies: prioritize larger, straighter vessels; avoid joint areas and damaged skin; if upper extremity vascular conditions are poor, consider lower extremity vessels; for long-term IV therapy patients, select unused vessels.
Technical operation points: needle insertion angles can be appropriately increased to 30-45 degrees; insertion should be decisive but not excessive; after successful puncture, stabilize needles preventing displacement; adequate vessel stabilization with thumb skin traction below puncture sites.
Post-procedure care emphasis: adequate compression time generally requiring 5-10 minutes; observe compression sites for bleeding or hematomas; for patients taking anticoagulants, extend compression time; instruct patients to avoid heavy lifting for 24 hours.
Safe Critical Patient Operations
Critical patient blood collection operations must consider not only technical issues but also safety and patient condition impacts.
Disease assessment importance: understand patient underlying diseases and current conditions; assess coagulation function status with coagulation indicator testing if necessary; understand medication use, especially anticoagulants; assess hemodynamic status determining collection appropriateness.
Collection timing selection: avoid patient unstable periods; if patients are using vasoactive drugs, proceed under physician guidance; for unconscious patients, select relatively quiet periods; coordinate timing with other treatment operations.
Technical safety points: strict sterile operations preventing nosocomial infections; gentle movements avoiding additional patient stimulation; closely monitor vital sign changes; immediately stop operations if patient discomfort occurs.
Special monitoring requirements: ICU patient collection requires monitoring equipment supervision; for ventilator patients, avoid affecting ventilation; record patient reactions and vital signs post-collection; notify attending physicians if necessary.
Emergency Situation Response Management
Emergency Management of Massive Bleeding
While massive bleeding from venipuncture is relatively rare, immediate effective management is required when it occurs.
Bleeding cause analysis: puncture injury to large vessels; patient abnormal coagulation function; anticoagulant medication use; vessel wall pathology such as aneurysms, arteriovenous malformations; inappropriate puncture site compression.
Emergency management steps: immediately use sterile gauze for compression hemostasis with uniform, moderate pressure; if routine compression is ineffective, use finger pressure hemostasis; elevate affected extremity reducing venous return; closely monitor bleeding volume and patient general condition; establish IV access and prepare for transfusion if necessary.
Prevention measure improvements: detailed pre-collection history taking understanding coagulation function; for patients with bleeding tendencies, select smaller collection needles; adequate post-collection compression hemostasis; for patients taking warfarin and other anticoagulants, extend compression time; establish bleeding risk assessment systems.
Syncope Management
Syncope is a relatively common complication during blood collection, more frequent in young females and first-time collection patients.
Early syncope recognition: patient pallor, cold sweats; complaints of dizziness, nausea; decreased blood pressure, weak pulse; consciousness confusion or loss.
Immediate management measures: immediately stop collection operations; place patient supine with head slightly lowered; loosen tight clothing maintaining airway patency; provide oxygen inhalation; measure blood pressure, pulse, and other vital signs; establish IV access if necessary.
Prevention strategy implementation: pre-collection inquiry about fainting history; provide psychological comfort for anxious patients; maintain comfortable collection environments with appropriate temperatures; operate skillfully and quickly; instruct patients not to hold breath, maintaining normal respiration.
Vasovagal Reaction Management
Vasovagal reactions are neurologic reflex responses manifesting as decreased blood pressure and bradycardia.
Clinical manifestation characteristics: patients suddenly develop pallor, nausea, vomiting; blood pressure drops sharply, potentially to 80/50mmHg; heart rate significantly slows, potentially to 40-50 beats/minute; patients may experience brief consciousness loss.
Management principles and methods: immediately place patient supine elevating lower extremities; administer atropine 0.5-1mg IM or IV push; monitor blood pressure and heart rate changes; if symptoms persist, consider vasopressor medications; consult cardiology if necessary.
Quality Control Points
Systematic Standardization Development
Standard Operating Procedure (SOP) Development and Implementation
Establishing comprehensive SOPs is fundamental for ensuring collection quality, requiring coverage of entire collection processes.
SOP development key points: clearly define specific requirements for each operational step; specify quality control critical points; establish abnormal situation management processes; create traceable record systems; regularly update and improve content.
Specific content framework: pre-collection preparation work checklists; collection technical points for different patient types; usage requirements for various collection tubes; specimen processing and storage standards; quality issue identification and management; equipment maintenance and management requirements.
Implementation guarantee measures: establish training and assessment systems ensuring all operators master SOPs; regularly inspect SOP implementation discovering and correcting problems promptly; establish quality supervision mechanisms implementing quality responsibility systems; collect feedback continuously improving SOP content.
Personnel Training System Construction
Professional skill training: basic theoretical knowledge training including anatomy, physiology, pathology foundations; practical operational skill training from basic movements to complex situation management; new technology and method training maintaining technical advancement; quality management concept training improving quality awareness.
Training method diversification: theoretical lectures combined with practical operations; case analysis and scenario simulation training; mentorship apprentice models; regular skill competitions and assessment evaluations; online learning platform applications.
Continuing education mechanisms: establish continuing education credit systems; attend professional academic conferences and training courses; encourage personnel to obtain relevant professional certifications; build learning organization culture.
Equipment Management and Maintenance
Vacuum Collection Tube Inventory Management
Procurement quality control: select qualified suppliers; establish supplier evaluation systems; conduct acceptance inspections for each batch; establish product quality files.
Storage environment management: dedicated storage areas avoiding direct sunlight; temperature and humidity control generally requiring 59-77°F temperature, 40-60% humidity; good ventilation avoiding harmful gases; classified storage with different types separated.
Inventory turnover management: execute first-in-first-out principles; regularly check expiration dates promptly handling expired products; reasonably control inventory levels avoiding accumulation; establish usage records tracking product quality.
Collection Equipment Maintenance
Daily needle holder maintenance: clean and disinfect after each use; regularly check spring mechanisms for normal function; inspect threaded connections for integrity; replace damaged units promptly.
Tourniquet management: dispose of single-use tourniquets immediately after use; reusable tourniquets require strict disinfection; regularly replace aging tourniquets; establish usage registration systems.
Auxiliary equipment maintenance: regular centrifuge calibration and maintenance; refrigerator temperature monitoring and recording; specimen transport box cleaning and maintenance; various disinfection equipment function checks.
Quality Monitoring Systems
Specimen Quality Indicator Establishment
Hemolysis rate control: establish hemolysis rate statistical systems; set hemolysis rate control targets (typically <2%); analyze hemolysis causes developing improvement measures; regularly summarize and report hemolysis situations.
Coagulation rate monitoring: track anticoagulant tube coagulation occurrence rates; analyze coagulation causes such as operational or equipment issues; establish coagulation prevention measures; investigate abnormal coagulation rate situations.
Specimen rejection rate statistics: establish specimen quality evaluation standards; track various non-conforming specimen proportions; analyze non-conformance causes developing improvement measures; regularly publish quality reports.
Continuous Improvement Mechanisms
Quality issue feedback systems: establish problem collection channels; promptly handle quality complaints; analyze root causes of problems; develop corrective and preventive measures.
Quality improvement projects: regularly conduct quality improvement activities; use quality management tools for problem analysis; develop quality improvement plans; evaluate improvement effectiveness.
Quality culture development: establish organization-wide quality awareness; recognize quality-advanced individuals and groups; create quality-pursuing cultural atmospheres; establish quality incentive mechanisms.
Safety Protection

Comprehensive Occupational Exposure Protection
Bloodborne Pathogen Exposure Prevention
Standard precaution principle implementation: treat all patient blood and body fluids as potentially infectious; adopt identical protective measures regardless of patient conditions; protective measures include proper use of personal protective equipment like gloves, protective clothing, face masks.
Personal Protective Equipment (PPE) selection and use: glove selection using disposable medical gloves, choosing appropriate sizes ensuring operational flexibility; protective eyewear or face shields preventing blood splatter to eyes and faces; protective gowns for potential extensive blood contact; respiratory protection using N95 masks for aerosol-generating procedures.
Correct donning and doffing sequences: donning sequence – first mask, then cap, followed by protective gown, finally gloves; doffing sequence – first gloves, then protective gown, followed by eyewear, finally mask; avoid facial and clothing contamination throughout the process.
Needlestick Injury Prevention and Management
Systematic needlestick injury prevention measures: never recap needles – this is the primary cause of needlestick injuries; use safety collection needles with automatic retraction or shielding functions; properly use sharps containers placing them near puncture sites; never handle contaminated sharps directly by hand.
Proper sharps container use: select appropriately sized sharps containers ensuring complete containment of used collection needles; sharps containers should not exceed 3/4 capacity; dispose of used collection needles directly into sharps containers without hand compression; place sharps containers within easy operator reach.
Post-needlestick injury emergency management: immediately squeeze wounds expressing contaminated blood; wash wounds with soap and water; disinfect wounds with povidone-iodine or 70% alcohol; immediately report and receive post-exposure prophylaxis; track contamination source patient infection status; consider prophylactic medication if necessary.
Strict Infection Control Implementation
Standardized Hand Hygiene Execution
Hand hygiene “Five Moments”: before patient contact; before clean or aseptic procedures; after body fluid contact; after patient contact; after patient environment contact. During collection operations, hand hygiene must be performed before and after each patient.
Standardized handwashing techniques: use running water and soap following seven-step handwashing method; handwashing time not less than 20 seconds; thoroughly rinse and dry hands; when no visible contamination exists, alcohol-based hand sanitizers may be used.
Hand skin care: regularly inspect hand skin integrity; use hand cream for skin protection; avoid wearing jewelry affecting handwashing effectiveness; temporarily discontinue collection operations when hand injuries exist.
Standardized Environmental Disinfection Management
Collection surface disinfection: wipe surfaces with disinfectant wipes after each patient; conduct comprehensive disinfection at work beginning and end; immediately handle and disinfect when blood contamination is discovered; regularly replace disinfection supplies.
Equipment disinfection specific requirements: disinfect needle holders after each use; dispose or disinfect tourniquets based on type; regularly clean and disinfect blood pressure cuff covers; process other reusable equipment according to disinfection technical standards.
Air quality management: maintain good ventilation in collection areas; regularly test air quality; use air purification equipment when necessary; control personnel movement reducing cross-infection risks.
Standard Medical Waste Management
Accurate waste classification: dispose of used collection needles and other sharps in yellow sharps containers; dispose of contaminated gauze, cotton swabs in medical waste bags; process disposable gloves and other protective equipment as medical waste; classify and dispose of general household waste separately.
Standardized medical waste collection: use dedicated medical waste bags and sharps containers; seal promptly without overnight storage; accurately complete medical waste labels; designate specific personnel for collection and transport.
Waste temporary storage and transport: medical waste temporary storage areas meet health requirements; scheduled transport without accumulation; establish transport record ledgers; select qualified disposal units.
Conclusion
Vacuum blood collection tubes technology, as fundamental technology in modern medical laboratory procedures, has extremely important significance for ensuring laboratory test results quality and patient safety through standardized application. Through this comprehensive guide’s detailed exposition, we can see that seemingly simple blood collection operations actually involve numerous technical details and quality control points.
Every operational step has scientific basis and quality requirements. From vacuum blood collection tubes selection to phlebotomy techniques mastery, from blood specimen processing to quality control, every detail oversight may lead to test result deviations, subsequently affecting clinical diagnostics and treatment decisions. Therefore, healthcare professionals must approach every blood collection operation with rigorous attitudes, strictly executing each step according to standards.
Quality control is a continuous improvement process. As medical technology continues developing and quality requirements continuously improve, phlebotomy techniques and quality management must also keep pace with the times. Healthcare institutions should establish comprehensive quality management systems, regularly evaluate and improve blood specimen quality, ensuring accurate and reliable testing services for patients.
Healthcare safety protocols are not only necessary for protecting healthcare professionals but also important measures for preventing healthcare-associated infections. Every person engaged in blood collection work should possess good safety protection awareness, skillfully master various protection techniques, and while protecting personal safety, also protect patient and colleague safety.
Humanitarian care cannot be overlooked either. While focusing on technical standards and quality control, we must also attend to patient feelings and needs. Through professional techniques and warm service, we can reduce patient suffering and fear, demonstrating healthcare personnel’s professional competence and humanitarian spirit.
The continuous advancement of medical technology provides us with better tools and methods, but the fundamental principles of safety, accuracy, and efficiency remain unchanged. Only by combining advanced technology with rigorous operational standards and warm humanitarian care can we truly provide patients with high-quality blood collection services, contributing to accurate diagnosis and effective treatment.
Every healthcare worker should remember that behind every blood specimen is a patient’s health and life. Our professional competence and responsible attitude directly affect patient diagnosis and treatment outcomes. Therefore, we must continuously learn, continuously improve, continuously pursue excellence, making every collection operation a demonstration of professional standards and manifestation of life respect.






