The Hidden Crisis Behind Needlestick Injuries
In the global medical device market, blood collection needles serve as fundamental consumables for clinical diagnostics, with annual consumption exceeding billions of units worldwide. However, according to World Health Organization statistics, healthcare workers experience millions of occupational exposure incidents from needlestick injuries annually, with blood collection procedures accounting for over 35% of these cases. This situation not only threatens healthcare worker safety but also imposes significant occupational protection costs and legal risks on medical institutions.
Against this backdrop, safety blood collection needle technology innovation has become an industry focal point. This article provides an in-depth analysis from market landscape, technological evolution, and product differentiation perspectives, examining how pen-type butterfly safety blood collection needles meet the triple demands of global healthcare facilities for safety, efficiency, and economy.
Why Safety Blood Collection Needles Have Become a Global Healthcare Procurement Imperative
Regulatory Drivers: Stricter Global Needlestick Injury Prevention Legislation
Since the enactment of the U.S. Needlestick Safety and Prevention Act in 2000, major markets including the EU, Japan, and China have successively introduced mandatory safety device usage regulations. The 2010 EU Council Directive 2010/32/EU explicitly requires healthcare institutions to prioritize procurement of sharps with safety devices, directly driving annual growth rates of 12% in Europe’s safety blood collection needle market.
China’s National Health Commission issued the Technical Operating Standards for Intravenous Therapy in Medical Institutions in 2019, similarly emphasizing prioritized use of safety-type venipuncture devices, opening a policy window for the domestic safety blood collection needle market.
Economic Considerations: Occupational Exposure Costs Far Exceed Product Price Differentials
Traditional perspectives consider safety blood collection needles more expensive than conventional needles, but lifecycle cost analysis reveals the opposite. CDC data shows that direct medical costs for a single needlestick injury (including blood testing, prophylactic medication, follow-up monitoring) average $3,000-$7,000, while indirect costs (lost work time, psychological counseling, potential litigation) may exceed $100,000.
In comparison, safety blood collection needle unit price premiums are only 1.5-2 times those of conventional needles, with bulk procurement costs even lower. For a 500-bed hospital performing approximately 200,000 annual blood draws, comprehensive adoption of safety products increases equipment expenditure by roughly $15,000 but avoids expected needlestick injury losses exceeding $200,000, demonstrating significant return on investment.
Technical Requirements: Complex Patient Populations Demand Enhanced Operational Convenience
With aging populations and increasing chronic disease patients, the proportion of difficult-draw populations with poor venous conditions (fragile vessels, significant retraction) continues rising. Traditional straight-needle blood collection needles show low success rates and increased patient discomfort in these scenarios, while butterfly wing designs combined with flexible catheter products provide more stable puncture angles and minimal tissue trauma, significantly improving the blood collection experience.

What Core Pain Points Do Pen-Type Butterfly Safety Blood Collection Needles Address?
Product Definition and Technical Architecture
Pen-type butterfly safety blood collection needles are innovative venipuncture devices integrating multiple advanced designs, with core technical features including:
Three Major Innovative Designs:
- Ergonomic Pen-Type Holder: Mimics writing instrument design, providing optimal 45-60 degree puncture angle control with one-handed precision insertion
- Butterfly Wing Stabilization Structure: Flexible wings (typically polyethylene material) conform to skin for secure fixation, reducing needle displacement risk, suitable for pediatric and geriatric populations
- Active Safety Mechanism: After blood collection completion, pressing or pulling triggers automatic protective sheath coverage of needle tip, irreversible locking physically prevents secondary injury
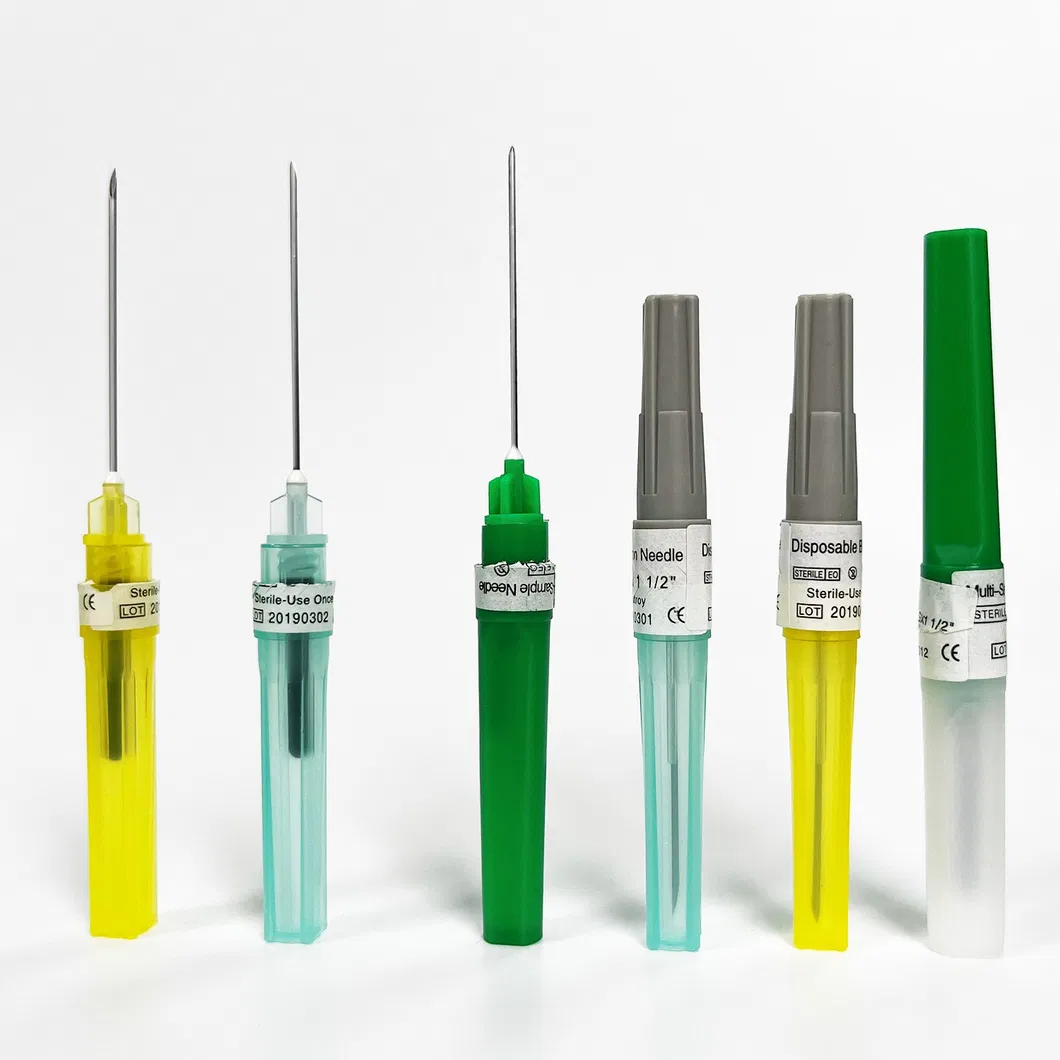
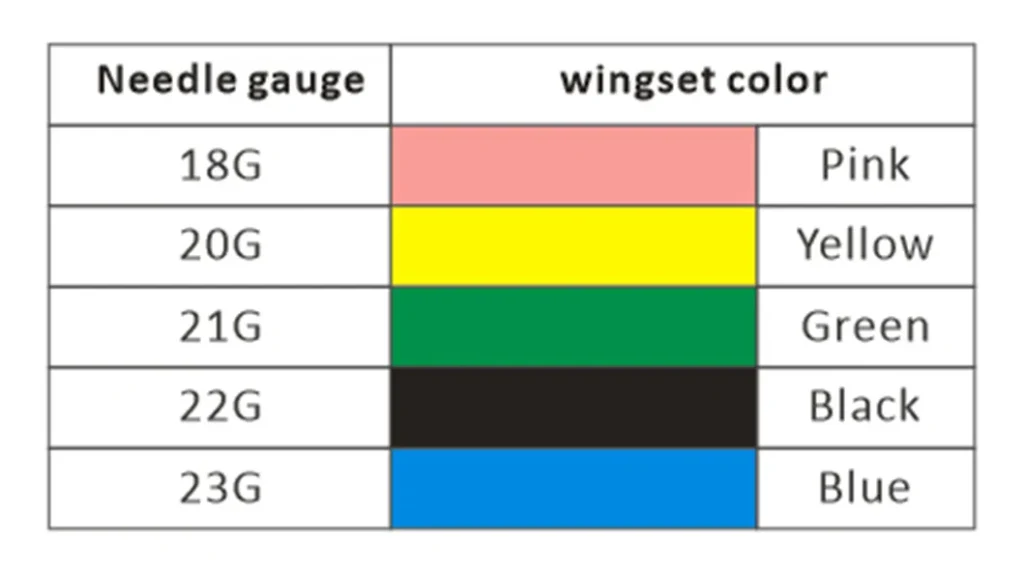
Comprehensive Specifications: 18G-23G Full Spectrum Meeting Differentiated Needs
Different clinical scenarios present distinct blood collection needle gauge requirements:
- 18G (Pink): Primarily for emergency transfusions and blood component collection—high flow rate but greater trauma
- 20G (Yellow): Adult routine blood draw preference, balancing flow rate and comfort
- 21G (Green): Most commonly used specification, suitable for most venous blood collection and infusion procedures
- 22G (Black): Appropriate for elderly patients or children with finer vessels
- 23G (Blue): Specialized for neonatal and hematology-oncology scenarios—minimal trauma
Professional manufacturers typically offer 18G-23G full-series custom manufacturing services, supporting combination wholesale orders based on actual institutional usage ratios to avoid inventory accumulation.
Materials and Sterilization Standards: Medical-Grade Quality Assurance
Products compliant with international standards must satisfy:
- Needle Tube Material: Medical-grade stainless steel (304 or 316L), surface-siliconized to reduce friction
- Catheter Material: Medical PVC or TPE, flexible and DEHP-free plasticizers
- Sterilization Method: Ethylene oxide (EO) sterilization, sterility assurance level (SAL) reaching 10^-6
- Packaging Requirements: Individual blister packaging, ready-to-use upon opening, 3-5 year shelf life
Who Are the Core Target Customers for These Products?
Medical Institution Segment
Hospital Laboratory and Emergency Departments: Daily blood collection volumes of 500-2,000 cases demand extremely high product stability and safety—the primary wholesale procurement force. These customers focus on: reducing healthcare worker occupational exposure risk, improving blood collection success rates, and optimizing patient satisfaction scores.
Blood Centers and Donation Stations: Large single-collection volumes (typically 200-400ml) require 18G-20G large-bore needles to ensure flow rates, while butterfly wing designs enhance donor comfort and reduce donation syncope incidence.
Primary Clinics and Health Examination Centers: Though blood collection volumes don’t match large hospitals, these facilities are price-sensitive, making wholesale procurement key to cost reduction.

Distributors and Distribution Channels
Regional Medical Device Dealers: Seeking manufacturers with OEM/ODM capabilities providing customized packaging and brand support for product differentiation.
E-commerce Platforms and Cross-Border Traders: Targeting overseas markets (Southeast Asia, Middle East, Africa), requiring products with CE, ISO 13485, FDA registration and other international certifications.
In Which Scenarios Do These Technologies Deliver Maximum Value?
High-Difficulty Puncture Scenarios
- Chemotherapy Patients: Long-term chemotherapy causes venous sclerosis and retraction; butterfly wing designs provide more stable fixation
- Neonatal Intensive Care Units (NICU): Small, fragile vessels require 23G micro-trauma needles with flexible catheters to minimize tissue damage
- Emergency Trauma Patients: Rapid venous access establishment; pen-type holders enable one-handed operation, freeing the other hand for additional emergency tasks
High-Volume Blood Collection Scenarios
- Blood Bank Donation Drives: Enhanced donor experience, reduced adverse reaction rates, increased return donation rates
- Corporate Health Screenings: Concentrated collection periods require efficient operations to reduce waiting times
- Epidemiological Survey Sampling: Such as COVID-19 antibody testing, requiring rapid, safe, standardized blood collection procedures
Home and Mobile Healthcare Scenarios
Portable design suits:
- Community health center home blood collection services
- Nursing home regular health monitoring
- Chronic disease patient home self-management (requiring professional training)
When Is the Optimal Window for Market Entry?
Policy Dividend Period
- 2024-2026: China’s medical insurance DRG/DIP payment reform deepening increases hospital cost control pressure, yet safety blood collection needles—given their clinical value in reducing complications and shortening hospital stays—may be included in priority procurement catalogs
- Sustained Growth in US-EU Markets: U.S. OSHA (Occupational Safety and Health Administration) regularly updates needlestick injury prevention guidelines, driving institutional procurement upgrades
Technology Iteration Cycle
Current mainstream safety blood collection needles have entered third-generation technology:
- First Generation (Pre-2000): Passive safety devices requiring manual activation—cumbersome operation
- Second Generation (2000-2015): Semi-automatic mechanical triggering, but some products exhibited false activation or failure risks
- Third Generation (2015-Present): Fully automatic activation + visual confirmation windows, combined with pen-type ergonomic design—market mainstream
Currently transitioning from third-generation maturity to fourth-generation (intelligent, digitalized) products, representing the golden period for scaled production and channel deployment.
How to Select Reliable Wholesale Manufacturers and Custom Solutions?
Manufacturer Qualification Audit Checklist
Professional procurement teams should prioritize verifying:
Certification Systems:
- ISO 13485 Medical Device Quality Management System
- CE European Certification (MDR 2017/745)
- FDA Registration (U.S. market access)
- China NMPA Registration Certificate (domestic market essential)
Production Capacity:
- Annual capacity meeting order demands (quality manufacturers typically exceed 100 million units annually)
- Class 100,000 cleanroom facilities availability
- Automated production line percentage (affects product consistency)
Quality Control:
- Batch-by-batch testing for needle sharpness, seal integrity, sterilization efficacy
- Product traceability systems provision (batch numbers, production dates, sterilization records)
Customization Service Capabilities
Packaging Customization:
- Customer brand LOGO printing support
- Multi-language instruction manual customization (English, Spanish, Arabic, etc.)
- Retail packaging and hospital bulk combination solutions
Specification Customization:
- Needle length adjustment (19-25mm options)
- Catheter length customization (standard 12 inches, extendable to 18 inches)
- Special model development (e.g., ultra-thin wall needles for enhanced flow rates)
MOQ and Lead Times:
- Minimum order quantities (quality manufacturers typically 50,000-100,000 units MOQ)
- Standard order delivery 15-30 days
- Emergency order expedited service capability
Cost Optimization Strategies
Volume Purchase Tiered Pricing:
- 50,000-100,000 units: Standard pricing
- 100,000-500,000 units: 5-8% discount
- 500,000+ units: 10-15% discount with negotiable payment terms
Long-Term Cooperation Framework Agreements:
- Lock annual procurement volumes, enjoy price protection policies
- Priority supply rights, avoiding stock shortage risks
- Complimentary technical support and usage training
Market Trends and Future Outlook
Technology Development Directions
Intelligent Integration:
- Built-in RFID chips enabling full product traceability
- Companion mobile apps recording blood collection operation data
- AI vision assistance systems guiding optimal puncture site identification
Material Innovation:
- Biodegradable materials replacing traditional plastics
- Nano-coating technology further reducing friction coefficients
- Antimicrobial materials minimizing infection risks
Market Competition Landscape
The global safety blood collection needle market exhibits a “Western technology leadership, Asian manufacturing emergence” pattern. Western brands (BD, Terumo) dominate high-end markets, but Chinese manufacturers—leveraging cost advantages and rapid response capabilities—are rapidly gaining market share in emerging markets across the Middle East, Africa, and Latin America.
Market research institutions predict the global safety blood collection needle market will grow from the current $1.5 billion to $2.5 billion between 2025-2030, representing a compound annual growth rate of approximately 10.8%, with the Asia-Pacific region showing fastest growth at over 15%.
Conclusion: Safety Blood Collection Needles as Rational Choices for Healthcare Cost Reduction and Efficiency Enhancement
Against the backdrop of continuously rising global healthcare safety standards, pen-type butterfly safety blood collection needles have transitioned from “optional configuration” to “standard configuration.” For medical institutions, selecting reliable wholesale manufacturers and establishing long-term customized cooperation relationships represents not only necessary measures for fulfilling occupational protection responsibilities but strategic investments in optimizing operational costs and elevating service quality.
Professional manufacturers should provide not merely products but serve as technical partners to healthcare institutions, jointly advancing venipuncture operations toward safer, more humanized directions through continuous product innovation, flexible customization solutions, and comprehensive after-sales services.
Learn more about wholesale and custom solutions for 18G-23G pen-type butterfly safety blood collection needles. Visit: Kohope Medical Product Page