Description
Detailed Technical Specifications
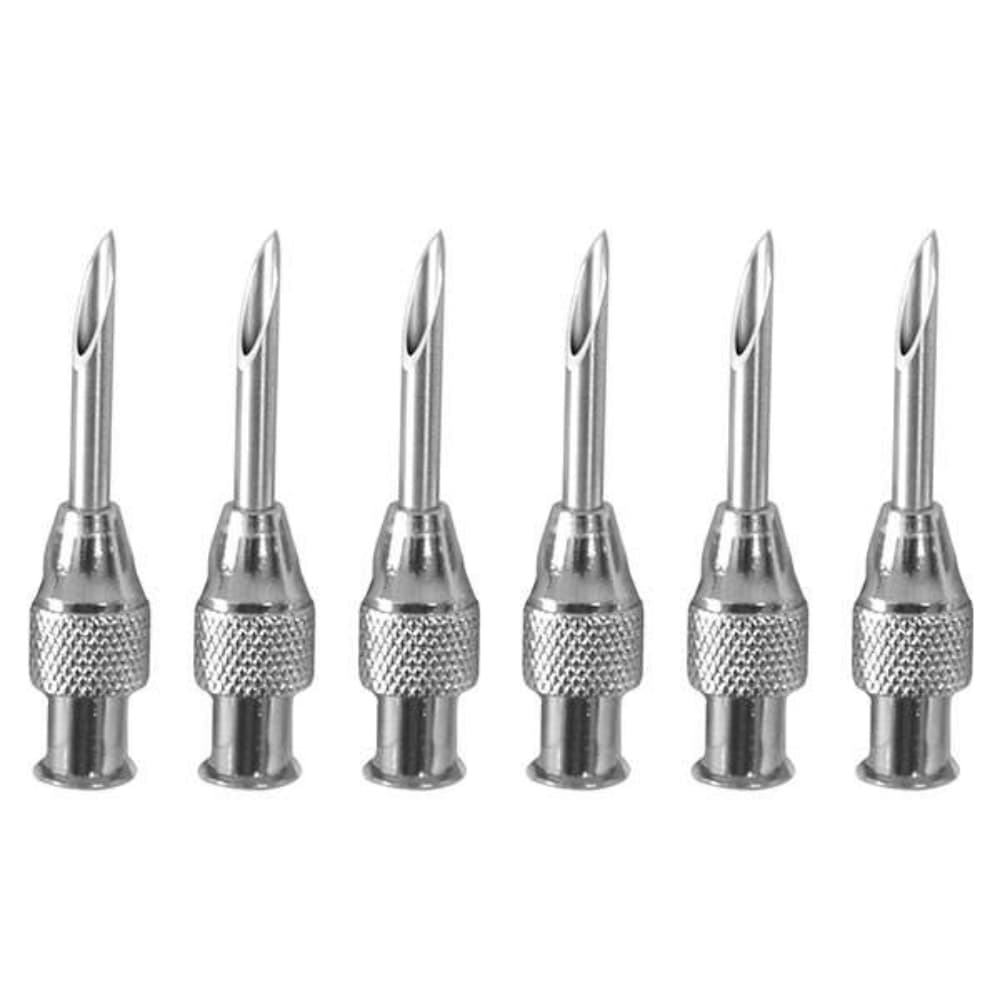
🔸 Needle Engineering
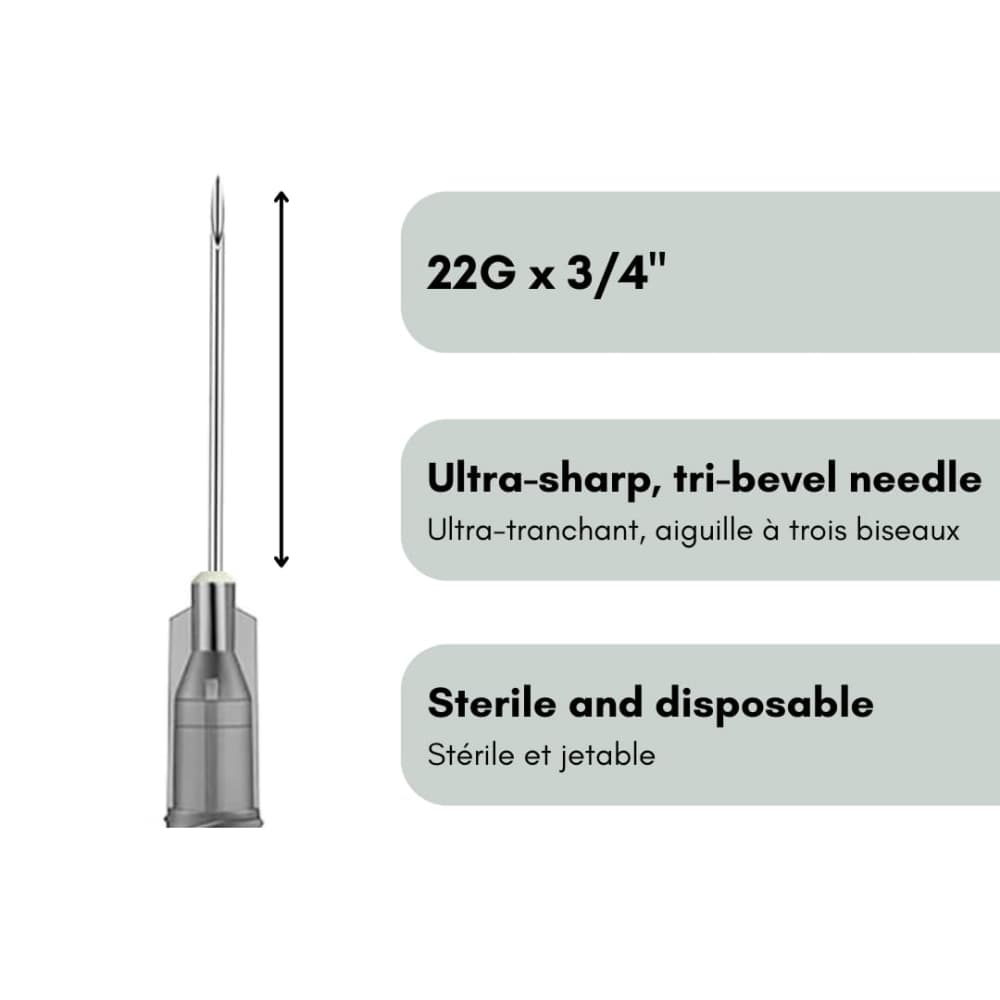
- Gauge Size: 22G (0.7mm outer diameter) – optimal medium gauge for most veterinary injection needles
- Needle Length: 3/4 inch (19mm) – standard depth for subcutaneous injection supplies
- Bevel Design: Tri-bevel cutting edge with optimized sharpness for minimal tissue trauma
- Tube Material: Medical-grade stainless steel with ultra-smooth surface finish for reduced friction
- Wall Thickness: Standard wall design ensures consistent fluid flow in veterinary hypodermic needles 22G
🔸 Hub Construction Features
- Material: Medical-grade polymer construction for disposable vet needles poly hub
- Color Coding: Industry-standard medical color identification for 22 gauge animal needles
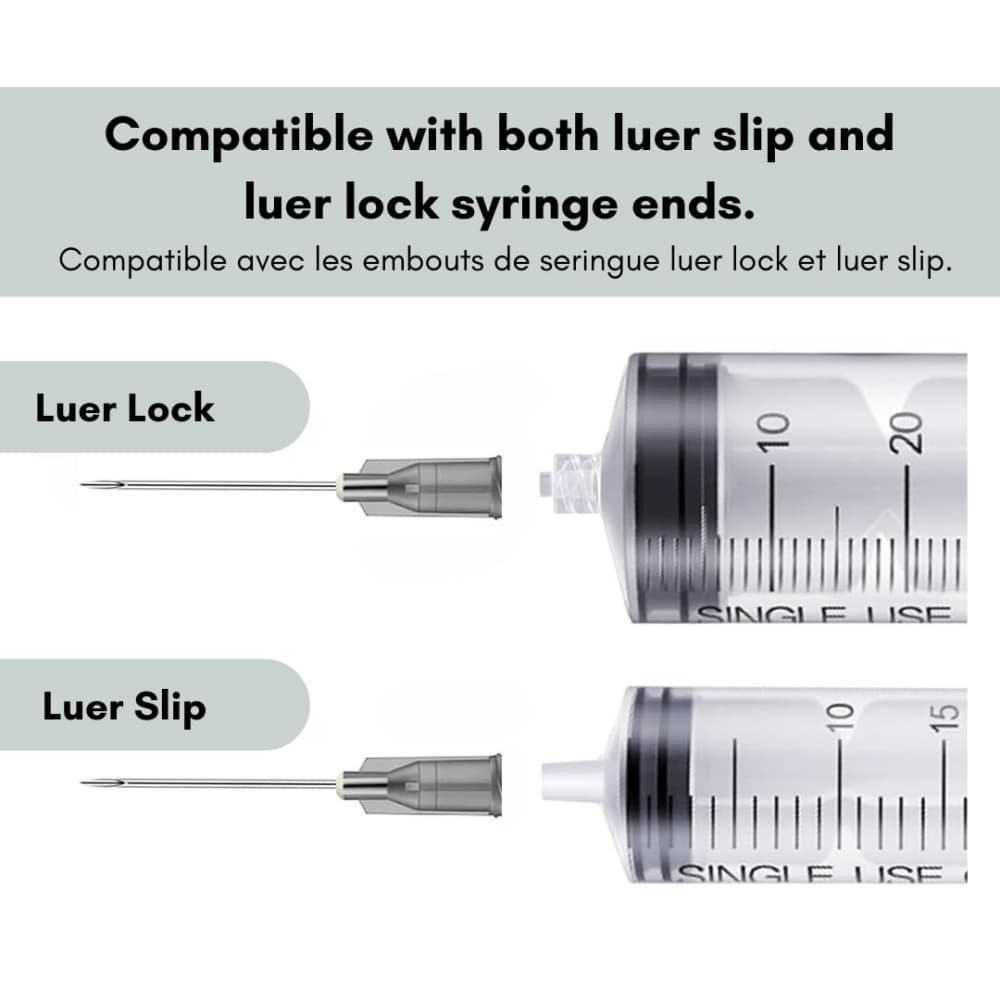
- Connection Interface: Universal Luer-Lok compatible fitting for seamless syringe compatibility
- Ergonomic Design: Human-engineered grip surface for secure handling and control
- Seal Integrity: Precision-molded construction ensures perfect syringe coupling without leakage
Packaging & Sterility Assurance
🔸 Multi-Layer Protection System
- Primary Packaging: Individual medical-grade paper-plastic pouch per needle
- Secondary Packaging: Medical-grade blister pack (Blister Package) for enhanced sterility
- Tertiary Packaging: 100-count medical storage box for organized inventory management
- Shipping Package: Moisture-resistant, tamper-evident transportation packaging
🔸 Sterility Technical Standards
- Sterilization Method: Ethylene Oxide (EO) gas sterilization process for sterile veterinary medical equipment
- Sterility Assurance Level: SAL 10⁻⁶ (one-in-a-million sterility assurance level)
- Package Integrity: Each package undergoes seal integrity validation testing
- Shelf Life: 5-year expiration from manufacturing date (unopened condition)
Animal Compatibility Analysis
🔸 Suitable Species & Weight Ranges
Small Breed Dogs (2-10kg/4-22lbs)
- Toy breeds: Chihuahua, Pomeranian, Yorkshire Terrier
- Small breeds: Beagle, Corgi, French Bulldog
- Needle length optimized for small breed subcutaneous fat layer thickness
Medium Breed Dogs (10-25kg/22-55lbs)
- Border Collie, Golden Retriever puppies, Shiba Inu
- Ideal for adult medium-breed subcutaneous injection requirements
Feline Patients (2-8kg/4-18lbs)
- All domestic cat breeds and mixed breeds
- Gauge specification particularly suitable for feline thin subcutaneous layer
- Designed to minimize stress response and improve patient cooperation
Exotic Small Animals
- Rabbits, ferrets, and similar companion animals
- Selected avian species for subcutaneous administration (requires professional assessment)
🔸 Injection Site Guidelines
- Canine Recommended Sites: Interscapular region, dorsal neck area with loose skin
- Feline Recommended Sites: Dorsal neck, interscapular loose skin areas
- Injection Angle: 30-45 degree angle relative to skin surface
- Penetration Depth: Subcutaneous fat layer, avoiding intramuscular injection
Clinical Applications

🔸 Vaccination Protocols
- Core Vaccines: Rabies vaccination, distemper combination vaccines
- Non-Core Vaccines: Kennel cough, feline combination vaccines
- Annual Boosters: Adult animal vaccination maintenance programs
- Puppy/Kitten Series: Primary immunization protocols for young animals
🔸 Therapeutic Interventions
- Antibiotic Therapy: Subcutaneous antibiotic formulations
- Hormone Administration: Insulin and other hormone replacement therapies
- Vitamin Supplementation: B-complex and other nutritional supplements
- Pain Management: Post-surgical analgesic administration
🔸 Preventive Healthcare
- Parasite Control: External parasite prevention injections
- Nutritional Support: Specialized nutritional supplement delivery
- Immune Modulation: Immune system enhancement protocols
Performance Advantages
🔸 Safety Excellence
- Zero Cross-Contamination Risk: Strict single-use design eliminates pathogen transmission
- Sterility Guarantee: Multi-layer packaging with EO sterilization ensures sterile condition
- Biocompatibility: Medical-grade materials prevent allergic reactions
- Sharpness Precision: Advanced manufacturing reduces tissue damage and patient discomfort
🔸 Operational Excellence
- Standardization: Complies with international veterinary standards for universal compatibility
- Visual Recognition: Color-coding system enables rapid gauge identification
- Ergonomic Comfort: Human-factor design reduces operator fatigue during extended procedures
- Secure Connection: Precision-engineered interface prevents disconnection during injection
🔸 Economic Value
- Bulk Packaging: 100-count packaging reduces per-unit acquisition costs
- Extended Shelf Life: 5-year expiration minimizes waste from outdated inventory
- Universal Specification: Standard gauge reduces SKU complexity and inventory investment
- Space Efficiency: Compact packaging design optimizes storage utilization
Technical Specification Chart
| Parameter | Specification | Standard Reference |
|---|---|---|
| Needle Outer Diameter | 22G (0.7mm) | ISO 9626 |
| Needle Length | 3/4 inch (19mm) | ±5% tolerance |
| Hub Material | Medical-grade polymer | USP Class VI |
| Sterilization Method | Ethylene Oxide | ISO 11135 |
| Package Count | 100 needles/box | – |
| Package Dimensions | 10.04×6.85×2.64 inches | – |
| Product Weight | 4.66 ounces | ±10% tolerance |
| Shelf Life | 5 years | Unopened condition |
Storage & Usage Guidelines
🔸 Storage Requirements
- Temperature Control: 59-86°F (15-30°C), avoid temperature extremes
- Humidity Control: Relative humidity ≤80%, prevent moisture infiltration
- Light Protection: Store away from direct sunlight in cool, dry environment
- Ventilation: Maintain adequate air circulation in storage area
- Stacking Protocol: Avoid compression, store in upright orientation per package arrows
🔸 Pre-Use Inspection Protocol
- Package Integrity: Verify outer packaging for damage or compromise
- Expiration Verification: Confirm manufacturing date and expiration status
- Needle Condition: Ensure needle shows no bending, corrosion, or defects
- Hub Inspection: Confirm hub shows no cracks, deformation, or damage
🔸 Proper Administration Technique
- Pre-Procedure Preparation: Hand hygiene, prepare alcohol swabs
- Aseptic Opening: Use sterile technique to open packaging
- Syringe Connection: Ensure secure attachment, verify no looseness
- Site Preparation: Disinfect injection site with povidone-iodine
- Angle Control: Maintain 30-45 degree needle insertion angle
- Controlled Injection: Slow, steady injection rate to minimize discomfort
- Safe Disposal: Immediate placement in approved sharps container
Quality Assurance & Certifications
🔸 Manufacturing Credentials
- Manufacturing License: Medical device manufacturing authorization
- Quality System: ISO 13485 Medical Device Quality Management System
- GMP Certification: Good Manufacturing Practice compliance certification
🔸 Product Certifications
- CE Marking: European Union Medical Device Directive compliance
- FDA Registration: US Food and Drug Administration device registration
- Veterinary Approval: Veterinary medical device specific certifications
🔸 Quality Commitment
- Product Warranty: 5-year quality guarantee under proper storage conditions
- Technical Support: 24/7 technical assistance hotline availability
- Problem Resolution: 100% satisfaction guarantee with quality issue replacement
Safety Information & Important Notices
🔸 Usage Precautions
- This product is intended for professional veterinary use only under licensed veterinarian supervision
- Strict adherence to single-use protocol required – reuse is strictly prohibited
- Pre-use verification of package integrity and product expiration is mandatory
- Proper injection site selection and technique must be employed
- Injection rate should be slow and controlled to prevent patient discomfort
🔸 Contraindications & Limitations
- Damaged packaging or expired products must not be used under any circumstances
- Animals with known material allergies should not receive injections with this device
- Infected sites or areas with dermatological conditions should be avoided
- Extra caution required in highly vascularized anatomical areas
🔸 Waste Management Protocol
- Used needles must be immediately disposed of in approved sharps collection containers
- Follow medical waste classification and disposal regulations
- Never dispose of as regular waste or attempt recycling
- Contract with licensed medical waste disposal services for proper handling
Regulatory Compliance
🔸 US Regulations
- FDA 21 CFR Part 820: Quality System Regulation compliance
- OSHA Bloodborne Pathogens Standard: Workplace safety compliance
- DEA Controlled Substance: Applicable for controlled substance administration
🔸 International Standards
- ISO 13485: Medical Device Quality Management Systems
- ISO 11135: Sterilization of healthcare products using ethylene oxide
- USP Class VI: Biological reactivity testing for medical devices
🔸 Veterinary Specific Regulations
- AVMA Guidelines: American Veterinary Medical Association standards
- State Veterinary Board: Individual state licensing and practice requirements
- Animal Welfare Act: Federal animal welfare compliance standards
Manufacturer Statement: This product is manufactured in strict accordance with international medical device standards. Each production lot undergoes rigorous quality testing and validation. To ensure product quality and safe usage, please purchase through authorized distributors and verify authentic product identification. For quality concerns or technical questions, contact our technical support department immediately for assistance.