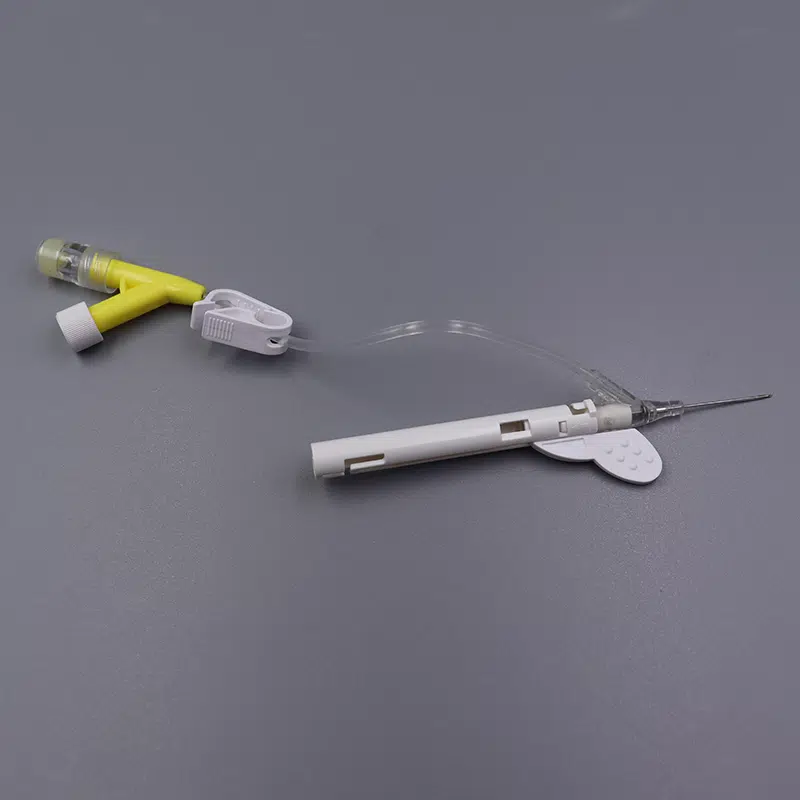
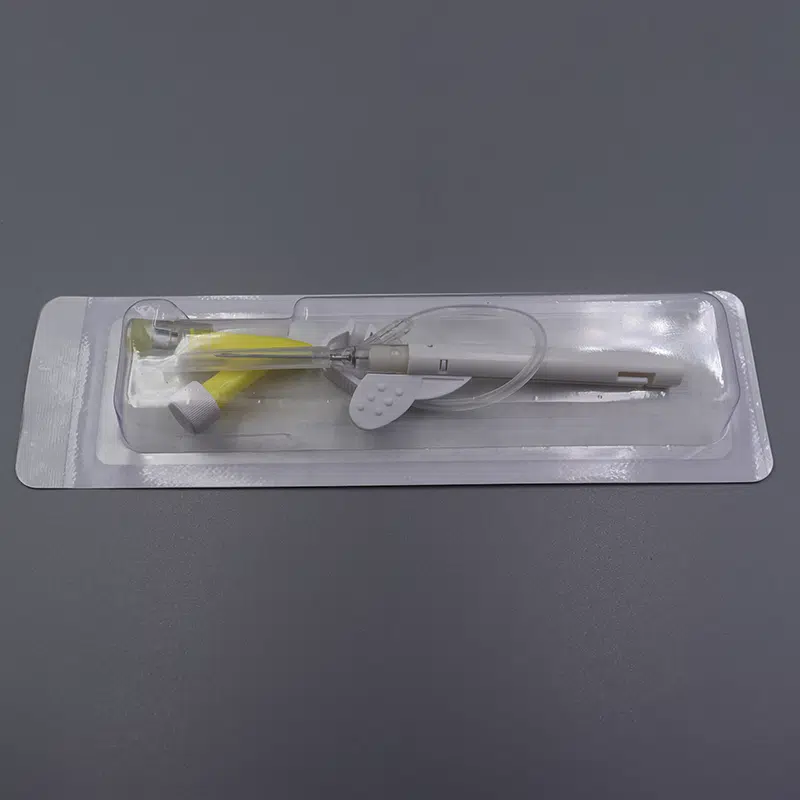
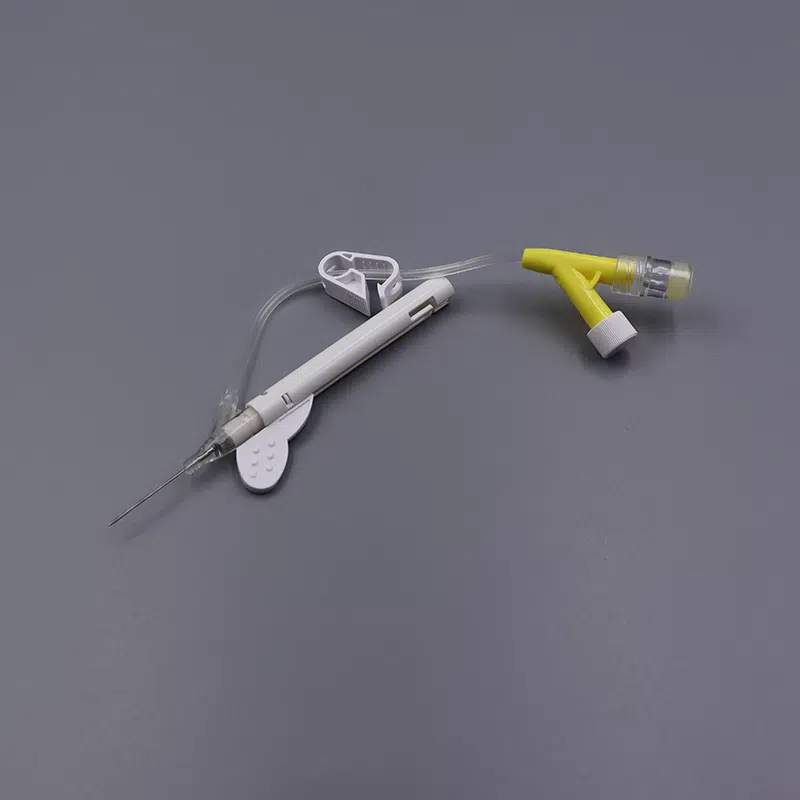
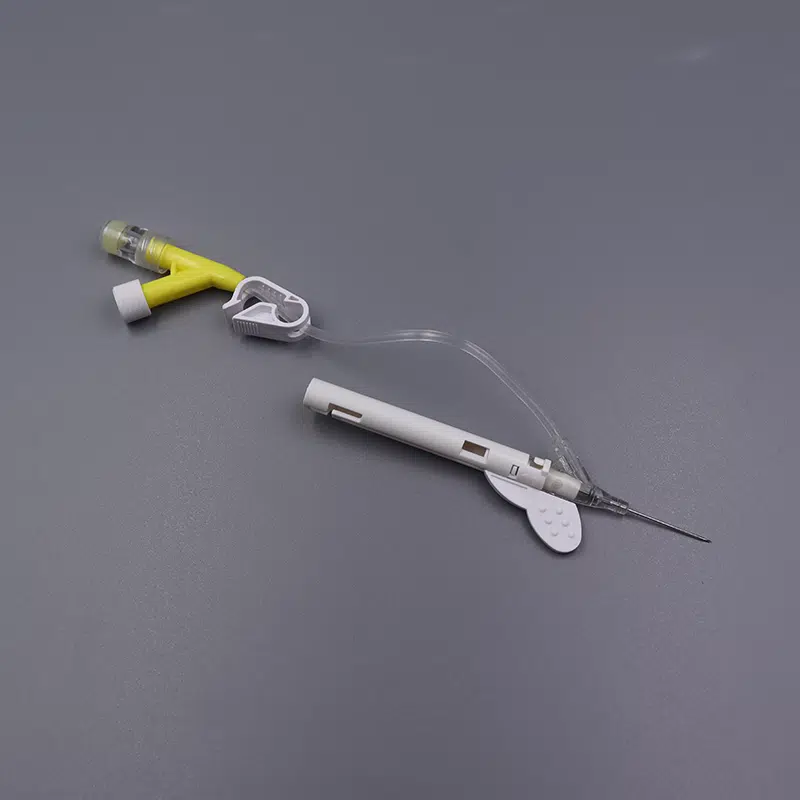
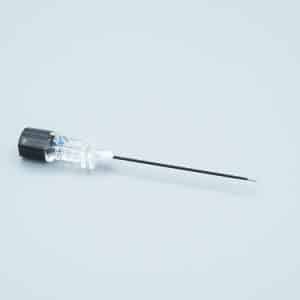
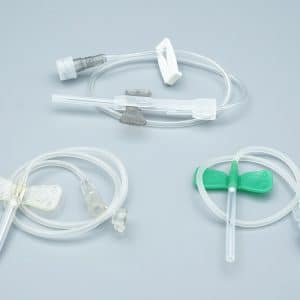
Description
Our Safety iv catheters Closed Y Type IV Cannula Catheter represents the next generation in intravenous access technology, combining passive safety features with advanced blood control mechanisms to deliver superior protection for healthcare professionals and patients alike.
Key Features & Benefits
Advanced Safety Technology
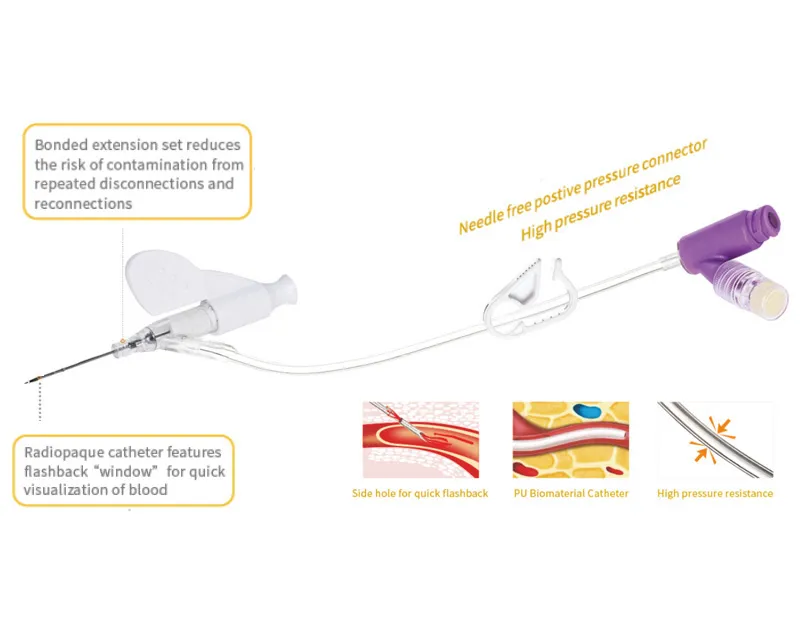
- Passive safety mechanism automatically activates upon withdrawal, eliminating the risk of accidental needle stick injuries
- Integrated blood control valve prevents blood exposure during catheter insertion and needle removal
- Compliant with international safety standards for healthcare worker protection
Superior Clinical Performance
- Universal Bevel Geometry: Engineered sharp needle design accommodates a wider range of insertion angles (15-45°), providing clinicians with enhanced flexibility and improved first-stick success rates
- Instant Flashback Visualization: Transparent “window” chamber delivers immediate blood confirmation, ensuring accurate vein placement and reducing patient discomfort
- PU (Polyurethane) Biomaterial Catheter: Medical-grade material offers exceptional biocompatibility, reduced thrombogenicity, and extended indwelling capability up to 96 hours
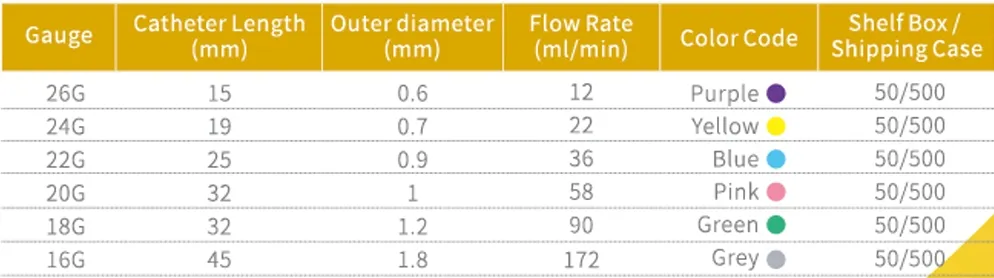
Complete Specification Range Available in comprehensive gauge sizes to meet diverse clinical requirements:
- 14GA – 26GA options
- Color-coded for quick identification
- Suitable for various patient populations from pediatric to adult care
Technical Specifications
| Parameter | Details |
|---|---|
| Product Type | Single/Dual Port Closed Y Type IV Cannula |
| Gauge Range | 14GA, 16GA, 18GA, 20GA, 22GA, 24GA, 26GA |
| Catheter Material | Medical-grade Polyurethane (PU) |
| Safety Feature | Passive activation mechanism |
| Blood Control | Integrated valve system |
| Sterilization | Ethylene Oxide (EO) |
| Shelf Life | 5 years |
Applications
Clinical Settings:
- Emergency departments
- Intensive care units
- Surgical procedures
- Chemotherapy administration
- Blood transfusions
- Long-term medication delivery
Household Care:
- Home healthcare services
- Palliative care
- Chronic disease management
Customization Services
Logo Printing Customize products with your brand identity. We offer:
- Silk screen printing on catheter hub
- Laser engraving options
- Custom packaging design
- Private labeling solutions
OEM/ODM Services
- Complete product customization
- Specification modifications
- Packaging design and production
- Quality system documentation support
Quality Assurance
- ISO 13485 certified manufacturing facility
- FDA registered products
- CE marked for European markets
- Strict quality control at every production stage
- 100% visual inspection and testing
- Comprehensive validation documentation
Ordering Information
- Minimum Order Quantity: 10,000 pieces
- Trademark Options: OEM or ODM
- Lead Time: 30-45 days after order confirmation
- Packaging: Individual sterile peel pouches, 50 pieces per box
- Shipping: Worldwide delivery available
Why Choose Our Safety IV Catheter?
- Enhanced Safety: Dual protection with passive safety and blood control reduces occupational exposure risks by 88%
- Clinical Excellence: Universal bevel design improves insertion success rates and patient comfort
- Extended Use: PU biomaterial allows longer indwelling time, reducing catheter replacements
- Cost-Effective: Reduced needle stick injuries lower treatment costs and liability exposure
- Regulatory Compliance: Meets international standards including ISO, FDA, and CE requirements
- Flexible Partnership: Comprehensive OEM/ODM services with competitive MOQ
Contact Us
Ready to enhance your medical supply portfolio with premium Safety IV Catheters? Contact our B2B sales team today for:
- Detailed product specifications
- Volume pricing quotations
- Sample requests
- Custom development inquiries
- Technical support and training
Protect your healthcare professionals. Improve patient outcomes. Choose Safety First.