Description
Understanding Your Procurement Challenges
Pain Point 1: Needle Coring and Port Damage Concerns Healthcare facilities frequently encounter costly port replacements due to coring damage caused by conventional needles. Standard needles can create silicone fragments that lead to port occlusion and patient complications, resulting in increased medical costs and liability risks.
Pain Point 2: Needlestick Injury Prevention Requirements With stringent workplace safety regulations, procurement managers face pressure to source safety Huber needle products that effectively minimize needlestick injury risks. Non-compliant products can lead to regulatory penalties and workers’ compensation claims.
Pain Point 3: Inconsistent Product Quality and Supply Chain Reliability Many distributors struggle with suppliers who cannot maintain consistent quality standards or reliable delivery schedules, causing inventory shortages and affecting patient care continuity.
Pain Point 4: Limited Customization and Certification Options Generic Huber needle products often lack the specific certifications, packaging configurations, or branding options needed for different regional markets and institutional requirements.

Why Choose Kohope Huber Needle Infusion Set
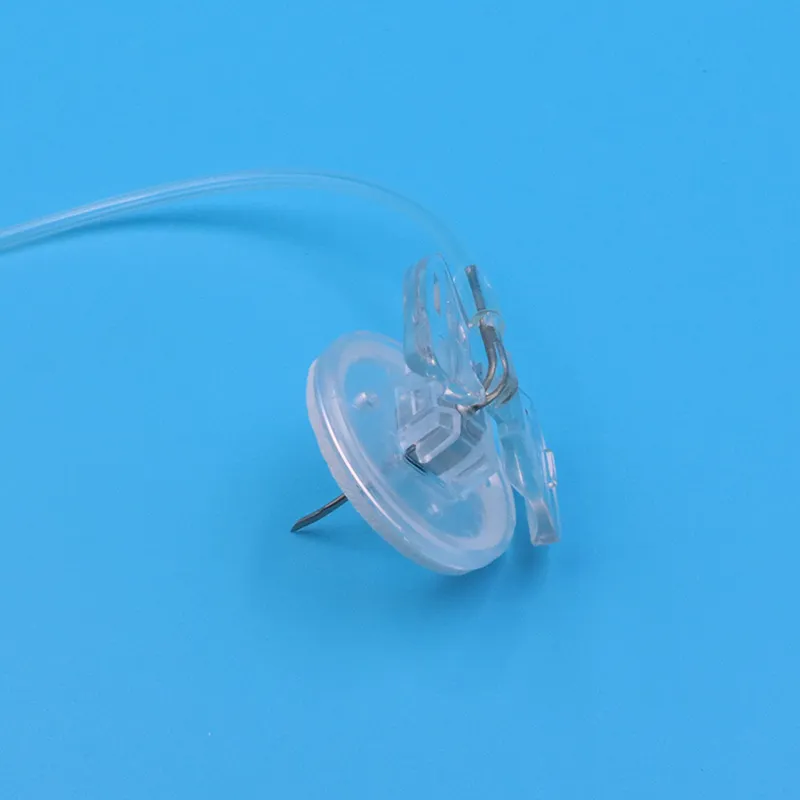
1. Advanced Non-Coring Technology
Our Huber needle infusion set features a precision-engineered deflected point design that prevents coring of silicone septum materials. The 90-degree angled tip cleanly separates septum fibers rather than cutting through them, extending implantable port lifespan by up to 300% compared to conventional needles.

Technical Specifications:
- Premium medical-grade stainless steel construction
- Ultra-smooth needle surface with polished finish
- Gauges available: 19G, 20G, 22G, 24G
- Lengths: 12mm, 19mm, 25mm, 32mm
- Color-coded safety caps for quick identification
2. Integrated Safety Lock Mechanism
Each safety Huber needle in our product line incorporates a spring-loaded protective shield that automatically covers the needle tip immediately upon withdrawal. This passive safety feature requires no additional user action, providing:
- One-handed activation for efficient clinical workflow
- Audible/tactile click confirmation of safety lock engagement
- Transparent shield allowing visual verification of proper activation
- Compliance with EU Directive 2010/32/EU and OSHA Bloodborne Pathogens Standard
3. Patient-Centric Design Features
Understanding that patient comfort drives purchasing decisions, our Huber needle infusion set includes:
- Ultra-thin wall technology: Maximizes flow rate while minimizing insertion trauma
- Soft, flexible extension tubing: 6-inch medical-grade PVC/polyurethane with clamp
- Low-profile butterfly wings: Secure adhesion with minimal skin irritation
- Transparent flashback chamber: Clear blood return visualization for accurate placement confirmation
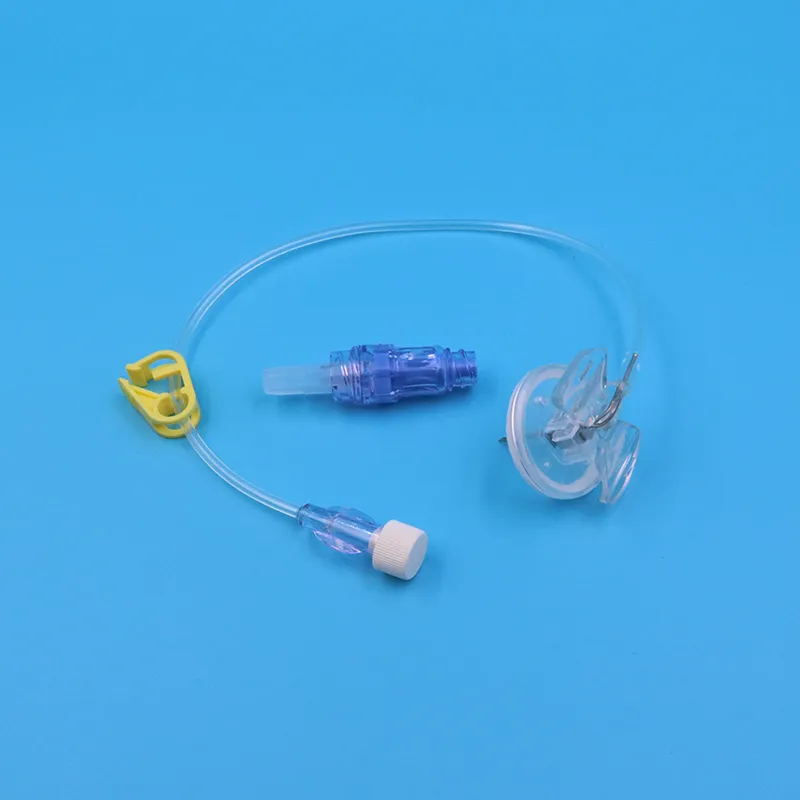
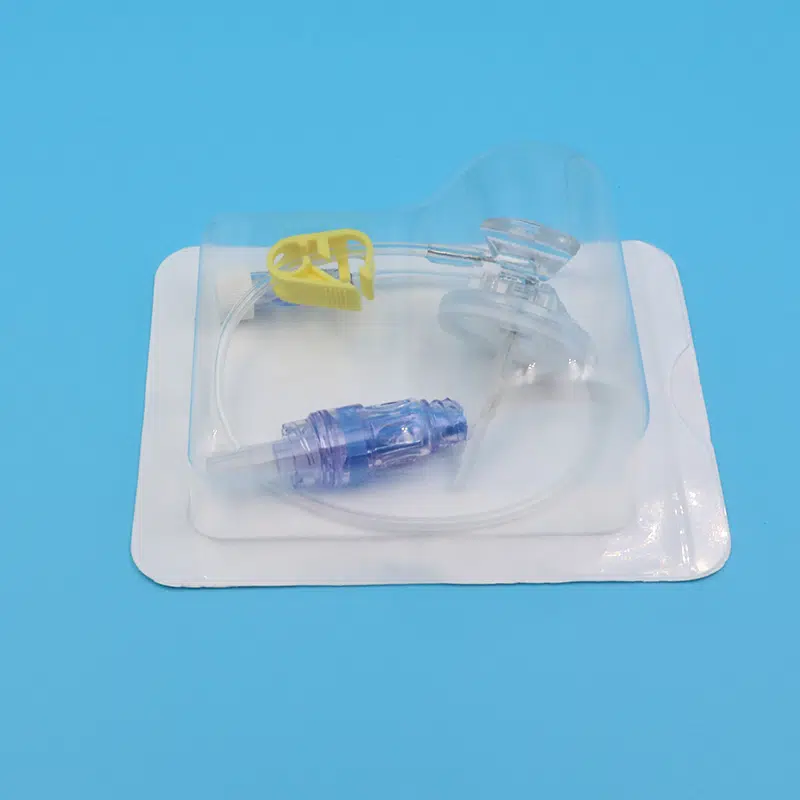
4. Complete Infusion Set Configuration
Each sterile Huber needle infusion set package contains:
- Pre-attached extension tubing with slide clamp
- Luer lock connector compatible with all standard IV systems
- Protective needle cap with built-in gripper
- Individual blister packaging with clear labeling
- 5-year shelf life with ethylene oxide sterilization
Quality Assurance and Certifications
Kohope Medical operates ISO 13485:2016 certified manufacturing facilities with comprehensive quality management systems. Our safety Huber needle products carry:
- FDA 510(k) Clearance: Registered Class II medical device
- CE Mark Certification: Compliant with EU MDR 2017/745
- ISO 80369-7 Compliance: Small bore connectors for intravascular applications
- Biocompatibility Testing: ISO 10993 complete evaluation
- Sterility Assurance Level: 10⁻⁶ (SAL) per ISO 11137
Every production batch undergoes rigorous inspection including:
- Needle sharpness and point geometry testing
- Safety mechanism activation force testing (3-8 Newtons)
- Leak integrity testing under pressure
- Residual ethylene oxide monitoring
- Package seal integrity validation
B2B Partnership Advantages
Flexible Order Quantities
- MOQ: 5,000 sets for standard configurations
- Sample orders available for evaluation
- Scalable production capacity up to 2 million units monthly
Custom Branding Solutions
- Private labeling with your company logo
- Custom packaging design and multilingual instructions
- Product modifications to meet specific market requirements
Competitive Pricing Structure Volume-based pricing for Huber needle infusion set orders:
- 10K-50K units: Tier 1 pricing
- 50K-200K units: Tier 2 pricing (8-12% discount)
- 200K+ units: Tier 3 pricing (15-20% discount)
Supply Chain Reliability
- Average lead time: 25-35 days for standard orders
- Safety stock program for key distributors
- Global shipping with full logistics support
- Real-time order tracking system
Clinical Applications
Our Huber needle products are specifically designed for:
- Chemotherapy drug administration via implantable ports
- Long-term parenteral nutrition (TPN) delivery
- Frequent blood sampling in oncology patients
- Contrast media injection for imaging procedures
- Continuous medication infusion protocols
Technical Support and Training
Kohope provides comprehensive B2B support services:
- Product training materials: Video demonstrations, usage guidelines, and clinical best practices
- Regulatory documentation: Complete device master files, biocompatibility reports, and certificates
- Marketing support: High-resolution product images, specification sheets, and promotional materials
- After-sales service: Technical consultation and complaint handling within 24 hours
Request for Quotation
Ready to enhance your medical supply portfolio with premium safety Huber needle products? Contact our B2B sales team today:
Inquiry Requirements:
- Desired product specifications (gauge, length)
- Estimated annual procurement volume
- Target market and certification requirements
- Customization needs (if applicable)
Our procurement specialists will respond within 12 business hours with detailed quotations, product samples, and partnership terms tailored to your distribution needs.
Why Global Distributors Choose Kohope
With over 15 years of experience in manufacturing Huber needle infusion set products, Kohope Medical has established partnerships with distributors in 45+ countries. Our commitment to quality, innovation, and customer service makes us the preferred supplier for:
- National hospital group purchasing organizations
- Regional medical equipment distributors
- Pharmacy chains and infusion centers
- Home healthcare providers
- International medical device trading companies
Partner with Kohope – where precision engineering meets patient safety in every safety Huber needle we manufacture.