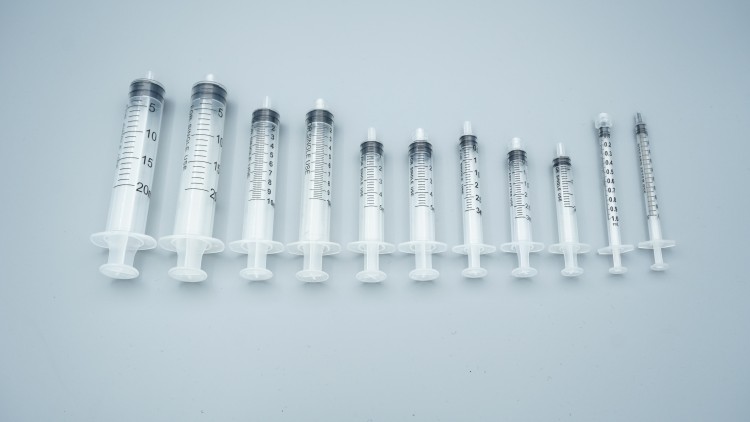
A tiny syringe—from plastic pellets to a safe, ready-to-use medical device—requires more than a dozen precision manufacturing steps. This seemingly simple product embodies sophisticated technologies in materials science, precision manufacturing, and quality control. This article demystifies how syringes are produced and explores the transformative changes reshaping this critical industry.
What: What Are Syringes Made Of? Why Do Materials Matter?

Core Components
A standard syringe consists of three primary parts:
The Barrel: This transparent tubular component features printed graduations. Think of it as a clear measuring cup—healthcare providers observe the liquid and read dosages through it. The material is typically medical-grade polypropylene (PP), a plastic offering excellent transparency and chemical stability that won’t react with medications.
The Plunger: This long rod pushes the medication. Its end features a rubber piston that functions like a bicycle pump piston, sealing and propelling the liquid forward.
The Rubber Gasket: This is the most critical component. It must be soft enough to conform to the barrel’s inner walls (preventing leakage) yet tough and durable enough to withstand repeated push-pull motions without deforming. Medical-grade silicone rubber is commonly used—similar in texture to baby bottle nipples.
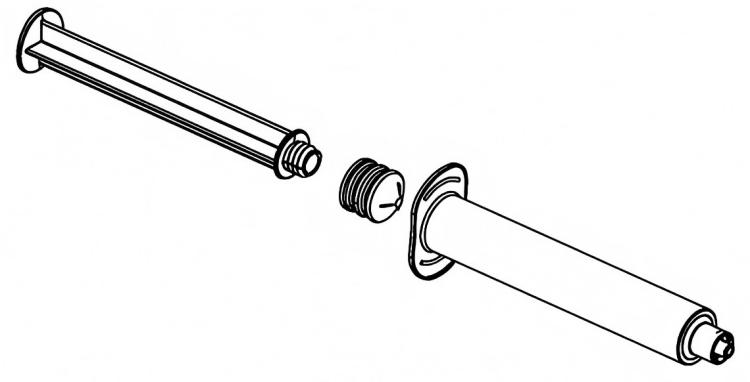
Why Materials Matter
Imagine if syringe materials contained toxic substances—these could leach into medications and enter the body. If the rubber is too hard, injection requires excessive force, potentially causing dosage inaccuracies. If materials can’t withstand high temperatures, they’ll deform during sterilization. Material selection directly impacts product safety and user experience.
Why: Why Is the Production Process So Complex? How Strict Are Quality Standards?
The Special Nature of Medical Devices
Unlike ordinary plastic products, medical syringes are classified as Class III medical devices (the highest risk category). The reason is straightforward: they directly contact blood and medications, and any quality issue could be life-threatening.
Examples of Stringent Requirements:
- Ordinary plastic bottles can tolerate several micrometers of error, but syringe graduation errors must remain within ±2%. For a 1mL syringe, error cannot exceed 0.02mL (about one-third of a water droplet)
- Ordinary products can be spot-checked, but syringes require 100% inspection—every single unit must pass multiple quality checkpoints
- Sterilization must achieve a 10⁻⁶ sterility assurance level, meaning the probability of one non-sterile unit among one million is less than 1
Regulatory Framework
Major global markets enforce strict regulations:
- US FDA requires 510(k) clearance or PMA approval
- EU requires CE certification (MDR regulations)
- China requires NMPA registration certificates
This explains why every production step is interconnected and cannot be compromised in the slightest.
Where: What Production Environment Is Required? How Important Is Cleanliness?

The Cleanroom Concept
Syringe production occurs in cleanrooms (also called dust-free workshops). What does this mean?
Relatable Comparison:
- Ordinary room: Contains millions of dust particles per cubic meter (invisible to the naked eye)
- Class 100,000 cleanroom: No more than 100,000 particles of 0.5 micrometers per cubic meter (equivalent to 1/200th of a hair’s diameter)
- Class 10,000 cleanroom: No more than 10,000 particles per cubic meter—the standard for syringe assembly workshops
- Class 100 cleanroom: Only a few hundred particles per cubic meter—the local environment at critical workstations
Why Such Cleanliness?

An invisible dust particle landing on a syringe’s inner wall could cause:
- Stuttering during injection (like sand in a bearing)
- Medication contamination
- Infection at the injection site
Practical Implementation:
- Workers must pass through air showers before entering (similar to airport air curtains, blowing attached particles off bodies)
- Wear full-body sterile suits, masks, gloves, and shoe covers, exposing only eyes
- Workshop maintains positive pressure (like “inflating” the room to prevent external air from entering)
- Installation of HEPA filters (capable of filtering 99.97% of 0.3-micrometer particles—equivalent to performing “dialysis” on air)
When: What Are the Critical Production Milestones? How Long Does Each Stage Take?
Production Timeline
Let’s follow a syringe’s journey from raw material to finished product:
Days 1-2: Raw Material Preparation (24-48 hours)
- Plastic pellet drying: 4-6 hours (removing moisture; otherwise bubbles form during injection molding)
- Raw material inspection: 1-2 days (testing purity, biocompatibility)
Days 3-5: Injection Molding (Core Process)
- Single barrel molding time: 3-5 seconds (yes, just seconds! But mold adjustment requires hours)
- Cooling and setting: 10-15 seconds
- Plunger molding: 5-8 seconds
- Daily output per injection molding machine: 100,000-200,000 units
Days 6-7: Printing and Assembly (Rapid Completion)
- Graduation printing: Instantaneous, but requires 30 minutes for ink curing
- Automatic assembly: 200-300 units per minute
Days 8-20: Sterilization and Aeration (Most Time-Consuming Phase)
- Ethylene Oxide (EO) Sterilization: 6-12 hours
- Aeration Period: 7-14 days (allowing toxic residual gas to dissipate to safe levels)
Additional Notes: Like a newly renovated house needing ventilation, syringes require “airing out” after sterilization. Ethylene oxide is a highly effective sterilant but toxic, and must completely dissipate. This is the most difficult timeframe to compress in the entire process.
Alternative Methods:
- Gamma Ray Sterilization: Takes only hours, no aeration needed, but equipment investment reaches tens of millions
- E-Beam Sterilization: Completes in minutes, but limited penetration depth
Day 21+: Quality Inspection and Release
- Finished product inspection: 3-5 days
- Stability testing: Conducted periodically
Total Duration: From material input to factory output, minimum 3 weeks, typically 4-6 weeks.
Who: Who’s Manufacturing? Who Are the Key Players in the Supply Chain?
Global Major Manufacturers
International Giants:
- BD (Becton, Dickinson and Company, USA): World’s largest, approximately 30% market share, established 1898, deep technical expertise
- Terumo (Japan): Renowned for precision manufacturing, industry-leading plunger force control
- B.Braun (Germany): European leader, strong safety syringe technology
Chinese Manufacturing Power:
- ShangHai Kohope Medical and other enterprises have entered the global supply chain
- China accounts for approximately 50%+ of global production capacity, though international brands still dominate the high-end market
Supply Chain Division
Upstream:
- Petrochemical companies (providing PP, PC and other raw materials)
- Medical rubber suppliers (providing silicone rubber, butyl rubber)
Midstream:
- Syringe manufacturers (the main subject)
- Mold manufacturers (precision molds determine product accuracy)
- Automation equipment suppliers (providing injection molding machines, assembly lines)
Downstream:
- Hospitals, clinics
- Pharmaceutical companies (prefilled syringe demand)
- Vaccination programs (such as COVID-19 vaccination consuming billions of units)
Example: During the COVID-19 pandemic, global syringe demand surged. In 2021, COVID-19 vaccination alone consumed 8-10 billion syringes, causing global supply shortages and making production capacity a bottleneck in vaccination speed.
How: How Is a Qualified Syringe Produced? What Are the Core Processes?
Step 1: Injection Molding—From Plastic Pellets to Components (Why Important?)

This is the “heart” of the entire process, similar to using a giant 3D printer to “extrude” plastic components.
Process Principles (simplified):
- Plastic pellets are heated and melted like sugar (temperature approximately 200-250°C)
- High pressure “shoots” molten plastic into precision molds (like pressing dough into a mooncake mold, but at 1,000 atmospheres pressure)
- Rapid cooling and setting (like jello solidifying)
- Mold opens, finished product removed
Critical Control Points:
| Parameter | Requirement | What Happens If Uncontrolled? |
|---|---|---|
| Mold Temperature | 40-60°C | Too high causes deformation, too low creates rough surfaces |
| Injection Pressure | 80-120 MPa | Insufficient pressure causes incomplete filling, excessive pressure creates internal stress (prone to cracking) |
| Cooling Time | Precise to 0.1 seconds | Insufficient cooling causes deformation upon removal, excessive time affects efficiency |
| Wall Thickness Uniformity | ±0.05mm | Non-uniformity causes uneven stress, potential rupture during injection |
Technical Challenges:
- Transparency control: Like making clear ice cubes, bubbles or impurities obscure graduations
- Graduation clarity: Molds must engrave fine graduation grooves (error not exceeding 0.01mm)
Step 2: Two-Shot Molding—Installing the “Seal Ring” on the Plunger (How Achieved?)
This is an ingenious process:
Process Demonstration:
- First injection: Create hard plastic rod (plunger body)
- Place rod into another mold
- Second injection: Inject liquid silicone rubber at rod’s end
- Rubber and plastic “fuse” together at high temperature (similar to chocolate coating on bread)
Why Two-Shot Molding? If adhesive bonding is used, problems may occur:
- Adhesive toxic residues
- Weak bonding, detachment during use
- Poor sealing
Two-shot molding achieves molecular-level bonding, essentially “welding” two materials together.
Quality Inspection:
- Tensile testing: Rubber-rod bonding strength must withstand ≥50N tensile force (equivalent to hanging 5kg weight without detachment)
- Sealing test: After assembly, pressurize underwater—no bubbles should appear
Step 3: Graduation Printing—Accurate to 0.01mL (How Ensure Precision?)
Process Selection:
Pad Printing (traditional method):
- Principle: Like stamping, silicone pad transfers ink from steel plate to syringe
- Advantages: Low cost, high speed (600-800 times per minute)
- Disadvantages: Ink may wear off
Laser Marking (advanced method):
- Principle: High-energy laser beam “burns” permanent marks on plastic surface
- Advantages: Never wears off, high precision (±0.01mm)
- Disadvantages: Expensive equipment (500,000-1,000,000 yuan per machine)
Quality Assurance:
- Machine Vision Inspection: Like taking an “ID photo” of each syringe, high-speed camera (detecting 200-300 units per second) photographs graduations, AI algorithms automatically identify:
- Are graduations missing?
- Is positioning offset?
- Are numbers clear?
- Defective products automatically rejected (like mechanical arms picking out bad apples on a conveyor belt)
Step 4: Automated Assembly—Precision Robot Dance (How Achieve High-Speed Assembly?)

Modern production lines achieve assembly speeds of 200-300 units per minute. How?
Assembly Process Visualization:
Barrel feeding → Visual positioning → Robotic arm grabs plunger → Insert into barrel
→ Torque monitoring (ensure proper insertion without damage) → Plunger force testing
→ Sealing inspection → Qualified products output / Defective products rejected
Key Technologies:
Torque Monitoring:
- Imagine using a screwdriver to tighten a screw—you feel resistance suddenly increase when tight
- Machine monitors assembly force in real-time through sensors (accuracy 0.01N)
- Too little force → Not seated properly; too much force → Component damage
- Qualified range: 5-15N (varies by specification)
Plunger Force Testing:
- After each syringe assembly, robot “simulates injection”
- Plunger pushed in at constant speed, measuring required force
- Standard: ≤20N (equivalent to pushing with 2kg force, easily operable by healthcare workers)
- Excessive plunger force indicates: rubber gasket too tight, inner wall burrs, misaligned assembly
Sealing Inspection:
- Negative Pressure Method: Extract internal air, check for leakage (like blocking the top of a straw while drinking—liquid won’t drop)
- Positive Pressure Method: Fill with pressurized gas, use high-sensitivity sensors to detect leakage
Step 5: Sterilization—Killing All Microorganisms (How Thoroughly Disinfect?)

Ethylene Oxide (EO) Sterilization (adopted by 90% of companies):
Principle: Ethylene oxide is a gas that can penetrate packaging and plastic, killing all microorganisms (including bacterial spores, the most resilient life forms).
Process:
- Pre-treatment: Place packaged products in sterilization chamber, create vacuum (remove air)
- Humidification: Inject steam, increase humidity to 60-80% (moist environment makes microbial cell walls more vulnerable)
- Sterilization: Fill with EO gas, temperature 50-60°C, maintain 6-12 hours
- Aeration: Transfer products to aeration room, ventilate 7-14 days, allowing residual EO to dissipate to safe levels (<10 ppm, equivalent to dropping one drop of ink in a swimming pool)
Why Not High-Temperature Sterilization?
- Plastic heat resistance limited (PP begins deforming at approximately 120°C)
- Rubber ages and hardens at high temperatures
Gamma Ray Sterilization (premium solution):
- Principle: Uses high-energy rays from cobalt-60 radiation source to kill microorganisms (similar to X-rays but higher energy)
- Advantages: Strong penetration, no residue, no aeration needed, continuous production possible
- Disadvantages: Large equipment investment (30-50 million yuan), requires radiation protection
Comparative Example:
- EO sterilization: Like fumigating a room with insecticide, requires ventilation to disperse odor
- Gamma rays: Like UV disinfection, instantaneous completion without residue
Step 6: Packaging and Quality Release—Final Safeguard (How Ensure Absolute Certainty?)
Packaging Requirements:
- Primary Packaging: Blister packaging (like transparent toothbrush clamshells) or medical paper-plastic pouches
- Packaging must maintain sterility for 5 years (sealing test: random inspection after 3 years, sterility pass rate ≥99%)
- Easy-tear design (healthcare workers can easily open while wearing gloves)
100% Online Inspection (already implemented):
- Package integrity: High-speed camera detects damage, inadequate sealing
- Label verification: OCR recognizes batch number, expiration date correctness
- Metal foreign object detection: X-ray scanning (preventing metal debris from falling in during production)
Pre-Release Quality Control:
- Sampling for complete testing (typically 0.1-0.5% sampling)
- Sterility test: Place products in culture medium, incubate at 37°C for 14 days, no microbial growth permitted
- Pyrogen test: Inject into rabbits, observe for fever response (ensuring no bacterial endotoxins)
- Only when all tests pass and quality manager signs off can products be released
How (Advanced): What Do Cutting-Edge Production Processes Look Like? Where’s the Black Technology?
1. Fully Automated Integrated Production Lines—The Prototype of Unmanned Factories
Traditional Mode vs. Smart Mode Comparison:
| Process | Traditional Mode | Smart Mode |
|---|---|---|
| Injection Molding | Manual machine adjustment, spot checking | AI automatic parameter adjustment, 100% visual inspection |
| Assembly | Manual or semi-automatic | 6-axis robots, real-time force sensor feedback |
| Packaging | Manual boxing | Robot vision recognition, automatic boxing |
| Inspection | Spot checking (2-5%) | 100% online inspection, AI defect recognition |
| Personnel Requirements | 100 people/10K capacity | 10 people/10K capacity (90% reduction) |
In-Mold Assembly Technology (cutting edge):
- Concept: Complete assembly directly inside injection mold
- Example: Moment barrel molding completes, robotic arm inserts plunger before mold release, assembly completed before demolding
- Advantages:
- High speed (eliminates handling time)
- High cleanliness (reduces exposure steps)
- High precision (mold positioning more accurate than robots)
Real-World Case: Some of BD’s production lines have achieved “raw materials in, finished products out,” with completely closed and automated intermediate processes—workers only monitor equipment and replace materials.
2. AI Quality Control—The “Inspector” with Eagle Eyes (How Achieve 99.99% Accuracy?)

Limitations of Traditional Manual Inspection:
- Human eyes tire easily (after 2 hours continuous inspection, miss rate increases 50%)
- Strong subjectivity (different people have inconsistent standards)
- Slow speed (maximum 30-50 units per minute)
AI Vision Inspection System:
Hardware Configuration:
- High-Speed Industrial Camera: Captures 200-300 photos per second (equivalent to 10 different angle photos per syringe)
- LED Multi-Angle Lighting: Illuminates from different directions, making tiny defects “nowhere to hide”
- Industrial Computer: Equipped with GPU, processes images in real-time
AI Learning Process:
- Data Annotation: Manual marking of 100,000+ images (this is qualified, that has bubbles, that has scratches)
- Model Training: Deep learning algorithms learn features (like teaching a child to recognize apples—after seeing many, they can identify them)
- Continuous Optimization: Daily new data continues training, algorithms become increasingly “intelligent”
What Can It Detect?
- Appearance Defects: Scratches, bubbles, black spots, deformation (minimum detectable 0.1mm defects)
- Dimensional Accuracy: Measures wall thickness, length, inner diameter (precision ±0.01mm)
- Graduation Quality: Whether graduation lines are clear, aligned, complete
- Assembly Status: Whether plunger properly inserted, whether misaligned
Accuracy Rates:
- Detection rate: 99.9% (among 1,000 defects, only 1 missed)
- False positive rate: <0.1% (among 1,000 qualified products, less than 1 misjudged)
Comparison: Manual inspection detection rate approximately 85-90%, false positive rate 5-10%.
3. Digital Twin Technology—The “God’s Eye View” of Virtual Factories (What Is It?)

Concept Explanation: Digital twin means building a “digital twin” for the real production line, completely replicating the production process on computer.
Relatable Analogy:
- Like playing a simulation management game (such as SimCity), but this “game” completely synchronizes with the real factory
- Every syringe produced on the real line, the virtual line simultaneously “produces” one
- All equipment parameters, temperature, pressure, speed synchronize in real-time
Practical Application Scenarios:
Scenario 1: New Product Development
- Traditional method: Make physical samples for testing, adjust if failed, cycle 3-6 months
- Digital twin: Simulate 100 parameter combinations in virtual environment, find optimal solution, cycle shortened to 1-2 months
- Example: To develop a new specification syringe (such as 0.5ml micro-volume type), test different wall thickness and rubber hardness combinations in virtual environment, find design with optimal plunger force
Scenario 2: Predictive Maintenance
- Traditional method: Regular equipment maintenance (such as shutdown inspection every 1 million units), possibly premature (wasting capacity) or delayed (sudden failure more troublesome)
- Digital twin: Real-time monitoring of equipment “health”
- Injection molding machine screw wear degree
- Mold fatigue status
- Hydraulic system pressure fluctuations
- AI predicts: “This equipment can run another 72 hours, recommend maintenance during night shift”
Scenario 3: Real-Time Optimization
- Raw material batch variations (like flour from different regions, different water absorption)
- Digital twin system detects declining product qualification rate
- Automatically adjusts injection temperature, pressure (like automatic transmission adjusting gears based on road conditions)
Implementation Cost and Benefits:
- Investment: 5-20 million yuan (software + sensors + computing platform)
- Returns: Equipment efficiency increase 15-25%, defect rate decrease 30-50%, typically break-even in 2-3 years
4. Blockchain Traceability—Each Syringe’s “ID Card” (How Ensure Data Reliability?)
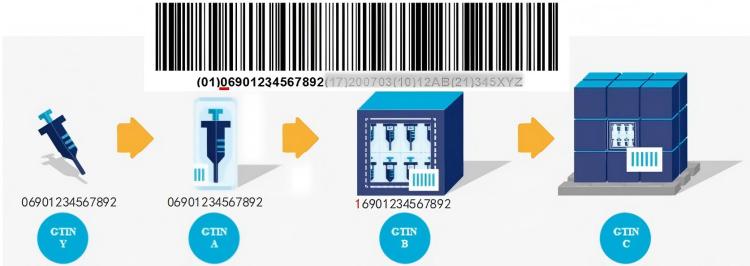
Pain Point: Traditional paper records may be tampered with; once quality incidents occur, companies may shirk responsibility.
Blockchain Solution:
Simplified Principle:
- Data from each production stage (raw material batch number, process parameters, inspection results) “broadcasts” like posting on social media
- All participants (raw material suppliers, manufacturers, logistics, hospitals) maintain a copy of the “ledger”
- No one can unilaterally modify unless everyone agrees (practically impossible)
Specific Implementation:
- Raw Material Warehousing: Scan QR code on material barrel, batch information goes on-chain
- Production Process: Injection molding machines, assembly machines automatically upload data
- Quality Inspection: Testing equipment automatically uploads results (no manual intervention possible)
- Logistics Distribution: Temperature and humidity records on-chain (ensuring qualified transportation conditions)
- Hospital Use: Scanning reveals complete history
Value Demonstration:
- Vaccination Safety: In 2021, a region experienced vaccination adverse reactions; through blockchain traceability, within 10 minutes identified which batch of syringes, which production line, what production time, quickly investigating causes
- Combating Counterfeits: Each product has unique digital identity, impossible to forge
Current Application Status:
- International: Some European and American companies piloting
- China: Shenzhen, Shanghai and other cities beginning use for high-end products (such as prefilled syringes)
Future Outlook: Where Is This Industry Heading? (What’s Next?)
1. New Materials Revolution—Breakthrough in Green Manufacturing

Biodegradable Materials:
Current Problem:
- Global annual syringe consumption approximately 20 billion units
- Post-use incineration produces dioxins and other harmful gases
- Plastic degradation requires 200-500 years
Solution: Polylactic Acid (PLA)
- Source: Plant starches from corn, sugarcane
- Degradation time: Complete decomposition into water and CO₂ within 3-6 months under industrial composting conditions
- Analogy: Like fallen leaves decomposing in soil, won’t permanently pollute environment
Technical Challenges:
- Strength: PLA approximately 25% softer than PP, prone to bending during injection
- Transparency: PLA slightly yellowish, affects medication observation
- Cost: Currently 50-80% more expensive than PP
Latest Developments:
- Modified PLA: Adding nanocellulose reinforcement (strength approaching PP)
- PHA Materials: Bacteria-synthesized bioplastics, better performance but higher cost
- Prediction: By 2030, bio-based materials may reach 10-15% market share
Nano-Modified Materials:
Antibacterial Syringes:
- Adding nano-silver or nano-copper particles to plastic (diameter 1-10 nanometers, virus-sized)
- Effect: Surface automatically kills bacteria on contact, 24-hour bactericidal rate >99%
- Application: Long-term hospitalized patients, reducing infection risk
Self-Lubricating Syringes:
- Depositing nano-coating on rubber surface (similar to Teflon non-stick pan coating)
- Friction coefficient reduced 60%
- Plunger force drops from 15N to 6N (easier for children, elderly patients)
2. Personalized Custom Production—3D Printing’s Disruptive Application
Current 3D Printing Limitations:
- Slow speed: Traditional 3D printing requires 30-60 minutes per syringe (injection molding only 3 seconds)
- High cost: Unit cost 10-20 yuan (injection molding cost 0.1 yuan)
Breakthrough Technologies:
Continuous Liquid Interface Production (CLIP):
- Principle: Uses UV light to solidify liquid resin layer by layer, but continuously rather than step-by-step
- Speed: 100 times faster than traditional 3D printing—one syringe in 20 seconds
- Application: Hospital-side printing, customized production for special patients
Example Scenarios:
- Pediatric Patients: Standard 1mL syringes too large, print 0.3mL micro-syringes on demand
- Special Medications: Certain drugs require special materials (such as light-sensitive drugs needing opaque barrels), small-batch customization
- Emergency Response: Disaster areas or remote locations with supply chain disruptions, on-site printing
Economic Viability:
- Current status: 3D printing suitable for small batches (<1,000 units), injection molding for mass production
- Future (by 2030): As technology matures, break-even point may drop to 10,000 units
3. Smart Syringes—From Passive Tools to Active Monitors
Integrated Sensor Technology:
Smart Injection Monitoring:
- Embedding micro-sensors in plunger (thickness only 0.5mm, won’t affect use)
- Real-time monitoring:
- Injection speed (too fast may cause pain, too slow drug may degrade)
- Injection pressure (detecting tissue resistance, preventing accidental arterial injection)
- Dosage accuracy (confirming actual injected volume)
- Data transmitted via Bluetooth to medical devices, automatically recorded in electronic health records
Drug Authentication:
- NFC chip embedded in barrel
- Scanning before injection verifies:
- Is medication genuine?
- Has expiration date passed?
- Are storage conditions qualified?
- Prevents counterfeit drug circulation, especially critical in developing countries
Patient Compliance Monitoring (for home injections):
- Recording injection time, dosage
- Reminding next injection time
- Data uploaded to cloud, allowing doctors to remotely monitor patient medication adherence
Market Prospects:
- Smart syringes currently 5-10 times more expensive than ordinary ones, mainly used for high-value biologics (such as insulin, growth hormone)
- As chip costs decrease, expected to enter mainstream market by 2028-2030
4. Sustainable Manufacturing—Circular Economy Model
Closed-Loop Recycling System:
Current Recycling Challenges:
- Medical waste regulations prohibit direct recycling (infection risk)
- Incineration wastes resources and pollutes
Innovative Solutions:
Chemical Recycling:
- Principle: Breaking down used syringes to molecular level, re-polymerizing into new plastic
- Process: Collection → High-temperature pyrolysis (400-600°C) → Purification → Repolymerization
- Outcome: Recycled plastic quality indistinguishable from virgin material
- Environmental benefit: Carbon emissions reduced 60% compared to producing from petroleum
Energy Recovery:
- For contaminated syringes unsuitable for material recycling, use advanced incinerators
- Heat generates electricity, supplying factories
- Exhaust gas passes through multi-stage treatment, emission standards exceed EU requirements
Case Study:
- A European company established pilot facility, processing 1,000 tons of waste syringes annually
- Recycled materials account for 30% of raw materials
- Goal: Achieving 50% recycling rate by 2030
5. Global Supply Chain Reshaping—Resilience and Localization
Lessons from COVID-19:
- Over-concentration in single regions created supply risks
- International logistics disruptions led to shortages
Future Trends:
Regional Production Hubs:
- Establishing production bases in major markets (Americas, Europe, Asia-Pacific)
- Each hub maintains 3-6 months strategic inventory
- Mutual backup in emergencies
Modular Mini-Factories:
- Developing containerized production units (20-foot container)
- Contains injection molding, assembly, sterilization equipment
- Deployable to disaster areas or underserved regions within 48 hours
- Suitable for rapid response to emergencies (pandemics, natural disasters)
Technology Transfer and Capacity Building:
- Developed countries assist developing nations in establishing local production capacity
- Through technology licensing, equipment supply, training programs
- Goal: Ensure every country has basic medical device self-sufficiency
Conclusion: The Convergence of Precision and Purpose
The journey from plastic pellet to life-saving medical device represents far more than manufacturing prowess—it embodies humanity’s commitment to healthcare excellence. As we’ve explored, every micron of precision, every second of sterilization, and every quality checkpoint serves a singular purpose: ensuring that when a healthcare provider reaches for a syringe, they hold an instrument worthy of absolute trust.
The future of syringe manufacturing stands at a fascinating crossroads. Traditional mass production continues to deliver billions of affordable, reliable devices globally, while emerging technologies promise revolutionary advances: biodegradable materials that honor our environmental responsibilities, AI systems that achieve near-perfect quality control, digital twins that optimize every parameter, and smart sensors that transform passive instruments into active health monitors.
Yet amid this technological renaissance, the fundamental principle remains unchanged: unwavering dedication to patient safety. Whether produced by century-old giants or cutting-edge smart factories, whether sterilized by established methods or revolutionary alternatives, every syringe must meet the same exacting standards.
The challenges ahead are significant—balancing cost with sustainability, scaling innovation while maintaining quality, and ensuring equitable global access. But the industry’s response to COVID-19 demonstrated its remarkable capacity for adaptation and resilience. When the world needed billions of additional syringes, manufacturers didn’t just meet demand; they innovated at unprecedented speed.
As we look toward 2030 and beyond, the convergence of green materials, intelligent manufacturing, and personalized medicine will reshape this essential industry. The humble syringe—humanity’s most ubiquitous medical tool—will continue evolving, becoming smarter, safer, and more sustainable. In this evolution lies not just industrial progress, but a profound commitment to global health and human dignity.
After all, behind every statistic, every production line, and every technological breakthrough stands a simple truth: somewhere, right now, a precisely manufactured syringe is delivering medication that saves a life, eases suffering, or prevents disease. That’s not just manufacturing—that’s purpose made tangible, precision serving humanity, one injection at a time.