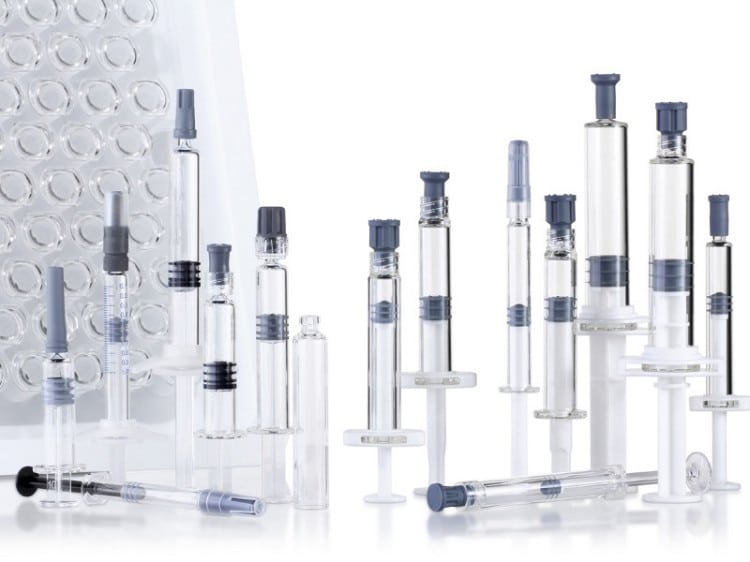
As a professional medical device manufacturer, we understand the critical role that pre-filled syringes (PFS) play in modern healthcare delivery. Pre-filled syringes have become the gold standard for drug delivery systems in biopharmaceuticals and vaccine administration across European and American markets. This comprehensive guide provides essential knowledge for healthcare professionals, wholesale distributors, and end-users throughout the EU and US markets.
What Are Pre-filled Syringes?
Pre-filled syringes are single-use injection devices pre-loaded with precise medication dosages. The system comprises a syringe barrel, pre-loaded medication, rubber stopper, needle (or needle adapter), and safety mechanisms. According to ISO 11040 standards, pre-filled syringes must meet stringent quality control requirements to ensure drug integrity and injection safety.
🔍 Key Technical Specifications
- Volume Range: 0.5mL to 20mL (Common sizes: 1mL, 2.25mL, 3mL, 5mL)
- Material Standards: USP Class I borosilicate glass or Cyclic Olefin Copolymer (COC)
- Accuracy Grade: ±2% (ISO 8537 compliant)
- Sterilization Methods: Ethylene Oxide (ETO) or Gamma radiation
- Storage Conditions: 2-8°C (refrigerated) or 15-30°C (room temperature)
⚠️ CRITICAL Pre-Use Preparation
1. Essential Product Inspection Checklist
Visual Inspection:
- ✅ Verify packaging integrity – no damage or contamination
- ✅ Confirm drug solution clarity – no precipitation or particles
- ✅ Validate label information (drug name, concentration, expiry, batch number)
- ✅ Check syringe barrel for cracks or defects
Technical Verification:
- ✅ Confirm medication not expired (typical shelf life: 18-36 months)
- ✅ Verify temperature storage records compliance
- ✅ Test safety device functionality
2. Environmental Preparation
- Clean, sterile operating environment
- Adequate lighting conditions
- Medical waste disposal container
- Required personal protective equipment

📋 Step-by-Step Usage Protocol
Step 1: Retrieval and Inspection
- Temperature Equilibration: If refrigerated, allow 10-15 minutes at room temperature
- Final Inspection: Re-confirm normal drug appearance and syringe integrity
- Hand Hygiene: Thoroughly wash hands or use alcohol-based sanitizer
Step 2: Syringe Preparation
- Remove Protective Cap: Gently twist and pull needle protection cap, avoid touching needle
- Air Bubble Elimination:
- Hold syringe vertically with needle pointing up
- Gently tap syringe barrel to move air bubbles upward
- Slowly push plunger until small amount of medication appears at needle tip
- Dose Confirmation: Verify accurate dosage through graduation marks
Step 3: Injection Site Selection
🎯 Recommended Sites:
- Deltoid muscle (upper arm) – preferred for vaccines
- Vastus lateralis (anterior thigh) – common for self-injection
- Abdomen (subcutaneous injections like insulin)
- Ventrogluteal (intramuscular injections)
Site Preparation:
- Disinfect skin with 70% isopropyl alcohol
- Disinfection area diameter: 5-8cm
- Allow disinfectant to air dry
Step 4: Injection Execution
- Skin Stabilization: Use non-injecting hand to stabilize injection site
- Needle Insertion:
- Intramuscular: 90-degree angle, rapid insertion
- Subcutaneous: 45-degree angle insertion
- Insertion depth: IM 12-25mm, SC 5-15mm
- Aspiration Check: Slight plunger withdrawal to confirm no blood return
- Medication Administration: Inject at 1-2mL/second rate
- Completion: Wait 2-3 seconds post-injection before needle withdrawal
Step 5: Post-Injection Protocol
- Needle Safety: Immediately activate safety mechanism to prevent needlestick injuries
- Hemostasis: Apply gentle pressure with alcohol swab for 2-3 minutes
- Waste Disposal: Dispose entire syringe in sharps container

💊 Drug-Specific Considerations
Biologics (Monoclonal Antibodies, etc.)
- Higher Viscosity: Requires larger gauge needles (21-23G)
- Injection Speed: Slower administration (0.5-1mL/second)
- Storage Requirements: Strict cold chain management (2-8°C)
Vaccines
- Standard Volume: Typically 0.5mL
- Needle Specifications: 22-25G, 25mm length
- Injection Site: Deltoid muscle preferred
Insulin
- Precision Dosing: Specialized insulin needles (29-32G)
- Injection Depth: Subcutaneous, 4-6mm depth
- Site Rotation: Prevent lipodystrophy
🏭 Quality Control & Traceability
Manufacturing Quality Standards
Our pre-filled syringes strictly comply with international standards:
- ISO 11040: Pre-filled syringe standards
- ISO 8537: Syringe accuracy requirements
- USP <1>: Injectable product quality standards
- Ph.Eur.: European Pharmacopoeia requirements
Batch Traceability System
Each product contains complete traceability information:
- Production batch number and date
- Raw material batch records
- Quality inspection reports
- Storage and transportation records

❓ Frequently Asked Questions
Q1: Can pre-filled syringes be reused?
A: ABSOLUTELY NOT. Pre-filled syringes are strictly single-use devices. Reuse poses contamination risks and inaccurate dosing.
Q2: How to handle a blocked needle?
A: If resistance occurs, do not force injection. Check for needle bending or blockage; replace with new pre-filled syringe if necessary.
Q3: What if medication shows precipitation?
A: Any precipitation, discoloration, or turbidity indicates potential drug degradation. Stop use immediately and contact supplier.
Q4: What about post-injection induration?
A: Mild induration is normal and typically resolves within 24-48 hours. Consult healthcare professionals if symptoms persist or worsen.

📦 Storage & Transportation Guidelines
Storage Conditions
- Temperature Control: Strictly follow label requirements
- Light Protection: Store in original packaging away from light
- Freeze Protection: Refrigerated products must not be frozen
- Humidity Control: Relative humidity 45-75%
Transportation Requirements
- Cold Chain: Use validated cold chain transportation systems
- Temperature Monitoring: Continuous temperature data logging
- Protective Packaging: Shock-resistant, damage-proof packaging design
- Time Control: Minimize transport time to ensure product quality
🏛️ Regulatory Compliance
US FDA Requirements
- 510(k) Clearance: All pre-filled syringes must obtain FDA approval
- cGMP Standards: Production processes comply with current Good Manufacturing Practices
- Quality System: ISO 13485 medical device quality management system
EU CE Certification
- MDR Regulation: Compliance with EU Medical Device Regulation 2017/745
- Technical Documentation: Complete technical files and risk analysis
- Clinical Evaluation: Adequate clinical data support
🚀 Innovation & Technology Development
Smart Syringes
- Electronic Monitoring: Digital injection process recording
- Dose Precision: Automated dose control systems
- Patient Feedback: Real-time injection status feedback
Biocompatible Materials
- Novel Polymers: Reduced protein adsorption
- Surface Treatment: Optimized siliconization
- Eco-friendly Materials: Biodegradable packaging materials

🎯 Key Takeaways
CRITICAL SUCCESS FACTORS:
✅ Proper Storage: Maintain cold chain integrity
✅ Thorough Inspection: Never use damaged or expired products
✅ Correct Technique: Follow 5-step protocol precisely
✅ Safety First: Always activate safety mechanisms
✅ Professional Training: Regular skill updates essential
🏆 Conclusion
Pre-filled syringes represent a cornerstone of modern medical delivery systems, with proper usage directly impacting treatment efficacy and patient safety. As professional manufacturers, we not only provide high-quality products but are committed to elevating industry standards through knowledge sharing and continuous innovation.
We recommend all users strictly follow this protocol and participate in regular professional training. For wholesale distributors, we provide comprehensive technical support and training services to ensure product quality and safety throughout the entire supply chain.
🏢 About Our Manufacturing Excellence
As a leading medical device manufacturer, we have specialized in pre-filled syringe research, development, and supply for over 20 years. Our products are distributed to 60+ countries worldwide, providing safe and effective drug delivery solutions for millions of patients. We are committed to continuous innovation and contributing to global healthcare advancement.
🌍 Global Certifications
- FDA 510(k) Cleared
- CE Mark Certified
- ISO 13485 Certified
- ISO 11040 Compliant
📞 Professional Support
For detailed product information or technical support, contact our professional team. We provide 24/7 technical consultation services to ensure safe and effective use of every pre-filled syringe.
Contact us today for wholesale inquiries and partnership opportunities.