Veterinary syringes are critical medical devices that directly impact animal health outcomes, treatment efficacy, and operational efficiency in veterinary practice and livestock management. This comprehensive guide provides essential knowledge for procurement professionals, covering everything from fundamental differences between veterinary and human-use syringes to advanced procurement strategies and market intelligence.
Part I: Understanding Veterinary Syringes – Essential Knowledge for Western Markets
1.1 What Makes a Veterinary Syringe Different?
Veterinary syringes are specialized medical devices engineered specifically for animal healthcare, vaccination programs, nutritional supplementation, and therapeutic treatments. Unlike human medical syringes, veterinary injection systems are designed to accommodate the unique physiological characteristics, behavioral patterns, and environmental challenges of animal healthcare.
1.2 Critical Physiological Differences
Skin Thickness Variations
- Cattle: 6-8mm (4x thicker than human skin)
- Swine: 2-4mm
- Sheep/Goats: 2-3mm
- Human Reference: 1.5-2mm
Muscle Architecture Impact Large animals (cattle, horses) require longer needle lengths (typically 25-50mm) to ensure accurate intramuscular injection delivery through thicker tissue layers, compared to human applications using 12-25mm needles.
Stress Response Considerations Animals exhibit heightened stress responses during medical procedures, demanding rapid injection techniques and ergonomically optimized veterinary syringe designs to minimize handling time and animal distress.

1.3 Can Human Syringes Replace Veterinary Syringes? – A Technical Analysis
Technical Feasibility Assessment
While human syringes may function for small companion animals in emergency situations, significant limitations exist:
🔴 Volume Constraints
- Human syringes: Maximum 50ml capacity
- Large animal dosing: Often requires 100-200ml single-dose volumes
- Cattle vaccination: Typical 5-10ml doses but multiple injections needed
🔴 Needle Specifications
- Human needles: 16-25G, 12-38mm length
- Large animal requirements: 14-18G, 25-50mm length for proper subcutaneous and intramuscular delivery
🔴 Material Durability Human syringes lack the mechanical strength to withstand large animal movement and potential impact forces during restraint procedures.
Regulatory Compliance Issues
- FDA (USA): Strict segregation between human and veterinary medical devices
- EU MDR: Veterinary medical devices require specific compliance pathways
- Risk Management: Cross-contamination and liability concerns
Economic Analysis
- Human syringes: $0.05-0.15 per unit
- Veterinary syringes: $0.08-0.30 per unit
- Total Cost of Ownership: Veterinary-specific products offer superior value when considering efficiency, safety, and regulatory compliance
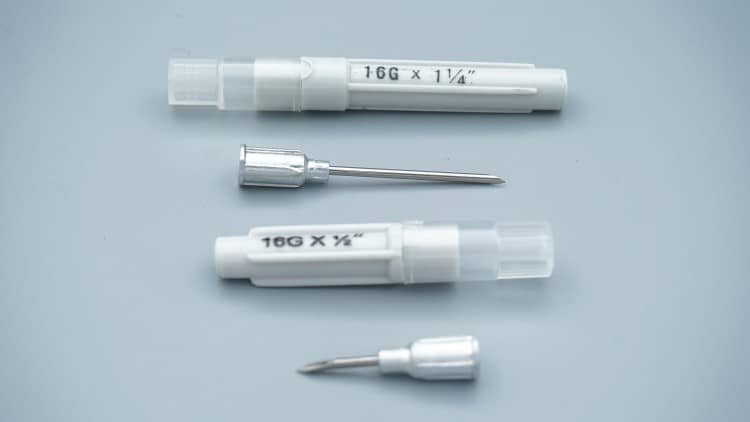
Part II: Veterinary Syringe Classification and Technical Specifications
2.1 Volume-Based Classification
🔹 Small Volume Syringes (1-10ml)
- Target Animals: Cats, rabbits, small dogs
- Primary Applications: Vaccination, precision medication
- Needle Specs: 23-25G, 13-25mm length
- Key Features: Luer-lock connections, low dead space
🔹 Medium Volume Syringes (10-50ml)
- Target Animals: Medium dogs, sheep, piglets
- Primary Applications: Routine treatments, nutritional supplements
- Needle Specs: 20-22G, 25-38mm length
- Key Features: Graduated markings, ergonomic grip
🔹 Large Volume Syringes (50-200ml)
- Target Animals: Cattle, horses, large swine
- Primary Applications: High-volume dosing, fluid therapy
- Needle Specs: 18-20G, 38-50mm length
- Key Features: Heavy-duty construction, anti-coring needles
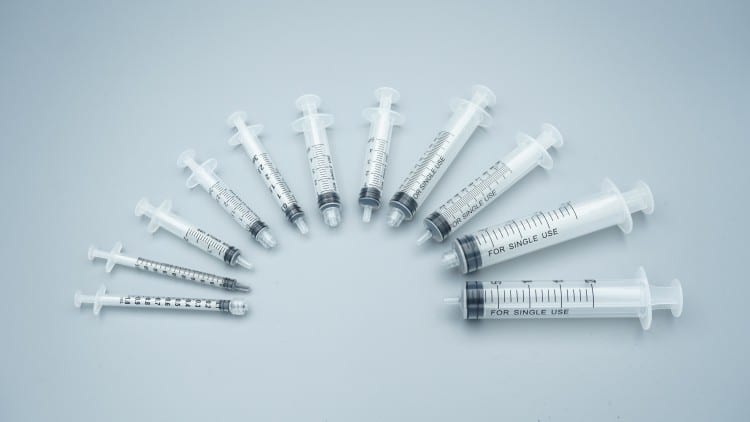
2.2 Material Technology Analysis
🔸 Polypropylene (PP) – Industry Standard
- Advantages: Excellent chemical resistance, cost-effective
- Limitations: Moderate transparency, static electricity issues
- Market Share: 45%
- Cost Range: $0.06-0.12 per unit
🔸 Polystyrene (PS) – Premium Clarity
- Advantages: Superior transparency, easy medication visualization
- Limitations: Brittleness, temperature sensitivity
- Market Share: 35%
- Cost Range: $0.08-0.15 per unit
🔸 Polycarbonate (PC) – High Performance
- Advantages: Maximum durability, autoclavable
- Limitations: Higher cost, complex manufacturing
- Market Share: 20%
- Cost Range: $0.12-0.25 per unit

2.3 Functional Categories
🔹 Disposable Syringes – Market Leader
- Market Share: 85%
- Key Benefits: Zero cross-contamination risk, convenience
- Applications: Vaccination programs, antibiotic therapy
🔹 Reusable Syringes – Sustainability Focus
- Market Share: 15%
- Key Benefits: Environmental sustainability, long-term cost savings
- Applications: High-volume commercial operations
🔹 Automatic Injection Systems – Growth Segment
- Market Share: 5% (15% annual growth)
- Key Benefits: Enhanced efficiency, reduced labor costs
- Applications: Large-scale livestock operations, mass vaccination programs

Part III: Global Veterinary Syringe Market Intelligence
3.1 Market Size and Growth Dynamics
📊 2023 Market Performance
- Global Market Value: $1.28 billion USD
- Annual Growth Rate: 8.3% CAGR
- 2028 Projection: $1.92 billion USD
🌍 Regional Distribution
- North America: 35% ($448M) – Premium product focus
- Europe: 28% ($358M) – Regulatory compliance driven
- Asia-Pacific: 25% ($320M) – Volume growth leader
- Rest of World: 12% ($154M) – Emerging opportunities
3.2 Market Drivers and Demand Catalysts
🐾 Companion Animal Market Expansion
- Global Pet Population: 984 million animals (2023)
- Growth Rate: 6.8% annually
- Impact: Increased demand for precision veterinary injection systems
🐄 Livestock Industry Modernization
- Commercial Farm Operations: 65% of global production
- Automation Adoption: 12% annual increase
- Efficiency Requirements: Driving high-volume syringe demand
💉 Vaccination Program Intensification
- Animal Vaccine Market: $11.0 billion USD
- Growth Rate: 7.5% annually
- Correlation: Direct impact on veterinary syringe consumption
3.3 Technology Evolution Trends
🔬 Smart Technology Integration
- IoT-Enabled Syringes: Real-time data collection
- RFID Tracking: Inventory and usage monitoring
- Dose Verification: Automated accuracy confirmation
🌱 Sustainability Revolution
- Biodegradable Materials: Bio-based polymer adoption
- Circular Economy: Recycling program implementation
- Carbon Footprint: Reduced packaging materials
📏 Standardization Progress
- ISO 7886 Veterinary Adaptation: Global standard development
- Interchangeability: Universal fitting requirements
- Quality Harmonization: Unified performance criteria

Part IV: Procurement Excellence for Veterinary Syringes
4.1 Supplier Evaluation Framework
✅ Essential Certifications
- ISO 13485: Medical device quality management
- CE Marking: European market compliance
- FDA 510(k): US market authorization
- GMP Certification: Good manufacturing practices
⚡ Production Capabilities
- Daily Capacity: Minimum 500,000 units
- Quality Control: <0.01% defect rate
- Lead Times: 7-15 days for standard products
- Customization: OEM/ODM capabilities
🔬 Technical Excellence
- R&D Investment: >5% of revenue
- Patent Portfolio: >10 active patents
- Technical Team: >20 qualified engineers
- Testing Equipment: Comprehensive quality validation systems
4.2 Product Selection Criteria
🎯 Performance Requirements
- Seal Integrity: <0.001% leakage rate
- Accuracy: ±2% (>10ml), ±5% (<10ml)
- Pressure Rating: >0.5 MPa
- Plunger Force: <15N operating force
🔧 Specialized Features
- Anti-Backflow Design: Prevents contamination
- Low Dead Space: <0.1ml residual volume
- Clear Graduations: Precise measurement visibility
- Ergonomic Design: Reduced user fatigue
📦 Packaging Standards
- Sterile Packaging: Individual or bulk sterile
- Moisture Protection: Barrier packaging
- Clear Labeling: Regulatory compliance
- Transport Safety: Damage-resistant packaging
4.3 Cost Optimization Strategies
📈 Volume Procurement Benefits
- 100K+ units: 5-8% discount
- 500K+ units: 12-15% discount
- 1M+ units: 18-22% discount
- Annual Contracts: Additional 2-3% savings
🔄 Supply Chain Optimization
- Regional Sourcing: 15-20% logistics cost reduction
- JIT Inventory: Reduced carrying costs
- Quality Assurance: Supplier warranty programs
- Payment Terms: 30-60 day credit arrangements

Part V: Quality Control and Standards Compliance
5.1 International Standards Framework
📋 ISO 7886-1 Standard
- Scope: Single-use sterile syringes
- Requirements: Material specifications, design parameters
- Testing Protocols: Standardized validation procedures
🇺🇸 ASTM Standards (USA)
- ASTM F2503: Syringe performance testing
- ASTM D6319: Package integrity validation
- ASTM F1929: Time-temperature indicators
🇪🇺 EN Standards (Europe)
- EN ISO 7886: Fundamental requirements
- EN 285: Sterilization validation
- EN 868: Packaging material specifications
5.2 Critical Quality Testing
🔍 Physical Performance
- Visual Inspection: Cracks, bubbles, deformation
- Dimensional Verification: Length, diameter, wall thickness
- Seal Integrity: Positive/negative pressure testing
- Plunger Performance: Force measurement testing
🧪 Chemical Analysis
- Material Identification: Infrared spectroscopy
- Heavy Metal Testing: Lead, mercury, cadmium limits
- Extractables Testing: Potential harmful substances
- Biocompatibility: Cytotoxicity validation
🦠 Microbiological Testing
- Sterility Assurance: Sterility testing protocols
- Endotoxin Testing: Bacterial endotoxin limits
- Package Integrity: Microbial barrier validation
5.3 Quality Assurance Systems
🏭 Supplier Quality Management
- Annual Audits: On-site facility inspections
- Quality Agreements: Defined performance standards
- Continuous Improvement: Quality issue tracking
- Risk Assessment: Supply chain risk management
📋 Incoming Quality Control
- Batch Testing: Statistical sampling validation
- Rapid Testing: Critical parameter verification
- Documentation: Complete quality records
- Non-Conformance: Standardized rejection procedures

Part VI: Application Case Studies and Best Practices
6.1 Large-Scale Livestock Operation
📊 Case Study: Commercial Swine Facility (100,000 head)
- Annual Syringe Demand: 5 million units
- Primary Specifications: 10ml, 20ml, 50ml volumes
- Procurement Strategy: Annual framework contract
- Cost Achievement: 25% unit cost reduction
📈 Performance Metrics
- Vaccination Efficiency: 40% improvement
- Labor Cost Reduction: 30% savings
- Animal Stress Reduction: 50% decrease in adverse reactions
6.2 Veterinary Hospital Chain
📊 Case Study: Multi-Location Companion Animal Practice (100 clinics)
- Annual Syringe Demand: 2 million units
- Primary Specifications: 1ml, 2ml, 5ml, 10ml precision syringes
- Quality Requirements: Human-grade medical device standards
- Special Features: Low dead space, Luer-lock connections
📈 Performance Outcomes
- Medication Waste Reduction: 60% decrease
- Injection Accuracy: 35% improvement
- Client Satisfaction: 20% increase in service ratings
6.3 Wildlife Conservation Program
📊 Case Study: International Wildlife Organization
- Specialized Requirements: Remote injection, dart syringes
- Unique Features: Large volume capacity, extended needle length
- Environmental Challenges: Extreme weather, field conditions
- Reliability Standards: Mission-critical performance
Part VII: Future Market Trends and Innovation
7.1 Technology Innovation Roadmap
🔬 Smart Technology Integration
- IoT Connectivity: Real-time data collection and analysis
- Animal Identification: Automated individual tracking
- Precision Dosing: Weight-based automatic adjustment
- Treatment Analytics: Efficacy monitoring and reporting
🧬 Advanced Materials
- Bio-Based Polymers: Sustainable, biodegradable options
- Nanotechnology: Antimicrobial surface treatments
- Smart Materials: Temperature-sensitive indicators
- Composite Engineering: Optimized strength-to-weight ratios
7.2 Market Growth Projections
📊 2024-2028 Forecast
- Market CAGR: 8.5% compound annual growth
- Premium Segment: Growth to 35% market share
- Automation Systems: 15% annual growth rate
- Sustainable Products: 25% market penetration
🌏 Emerging Market Opportunities
- Asia-Pacific: 12% annual growth
- Latin America: 10% annual growth
- Africa: 8% annual growth
- Middle East: 9% annual growth
7.3 Industry Challenges and Strategic Responses
⚠️ Market Challenges
- Raw Material Volatility: Price fluctuation management
- Regulatory Intensification: Compliance cost increases
- Technology Disruption: Rapid innovation cycles
- Competition Intensification: Market consolidation pressures
🚀 Strategic Opportunities
- Pet Economy Growth: Premium product demand
- Livestock Modernization: Automation integration
- Vaccine Expansion: Increased immunization programs
- Market Globalization: International expansion potential
Executive Recommendations
🎯 For Procurement Professionals
- Strategic Partnership Development: Establish long-term relationships with qualified suppliers demonstrating consistent quality and innovation capability
- Comprehensive Quality Systems: Implement robust quality validation processes with statistical process control and supplier scorecards
- Technology Roadmap Alignment: Stay informed about emerging technologies and plan for future integration requirements
- Supply Chain Resilience: Develop diversified supplier networks to mitigate risk and ensure continuity
- Total Cost Optimization: Focus on total cost of ownership rather than unit price, considering efficiency, safety, and regulatory compliance
🏭 For Manufacturing Partners
- Innovation Investment: Allocate significant resources to R&D for next-generation veterinary syringe technologies
- Quality Excellence: Implement world-class quality management systems exceeding international standards
- Capacity Expansion: Scale production capabilities to meet growing global demand
- Service Enhancement: Develop comprehensive technical support and customer service capabilities
- Global Market Access: Pursue international certifications and regulatory approvals for market expansion
Conclusion
The veterinary syringe market represents a dynamic, high-growth sector driven by pet industry expansion, livestock modernization, and vaccination program intensification. Success in this market requires deep understanding of animal physiology, regulatory requirements, and emerging technologies.
Key Success Factors:
- ✅ Product Differentiation: Specialized designs for specific animal applications
- ✅ Quality Assurance: Uncompromising commitment to safety and efficacy
- ✅ Innovation Leadership: Continuous technology advancement
- ✅ Market Intelligence: Data-driven decision making
- ✅ Partnership Excellence: Collaborative supplier relationships
The future belongs to organizations that can combine technical excellence with market insight, delivering veterinary syringe solutions that enhance animal health outcomes while optimizing operational efficiency and cost-effectiveness.
Ready to transform your veterinary syringe procurement strategy? Contact our technical experts for customized solutions and market intelligence tailored to your specific requirements.