In today’s healthcare system, laboratory testing has become the cornerstone of disease diagnosis, treatment monitoring, and prognostic evaluation. Statistics show that over 70% of medical decisions are based on laboratory test results. However, many healthcare professionals may not realize that something as seemingly simple as selecting the right blood collection tubes can directly impact diagnostic accuracy and treatment outcomes. Understanding the different types of clinical blood collection tubes and their specific anticoagulant mechanisms is crucial for ensuring optimal specimen quality control.
Picture this scenario: An emergency department receives a 65-year-old patient suspected of having a stroke. The physician urgently orders coagulation studies. In the rush, a nurse grabs a purple EDTA tube for blood collection. When the sample reaches the laboratory, it’s rejected as unsuitable, requiring a new blood draw. This not only delays the patient’s care but also increases patient discomfort and healthcare costs. Such situations occur regularly in clinical practice, and the root cause is often a lack of clear understanding of blood collection tube types and their applications.
This comprehensive guide will take you through everything you need to know about clinical blood collection tubes, ensuring you can make the right choice every time while following phlebotomy best practices.
Why Must We Distinguish Different Types of Blood Collection Tubes?
The Complexity of Blood Specimens
Human blood is an extraordinarily complex biological system containing cellular components like red blood cells, white blood cells, and platelets, along with plasma constituents including various proteins, enzymes, hormones, and electrolytes. Once blood leaves the circulatory system, it undergoes a series of physiological and biochemical changes.
First is the initiation of coagulation. Under normal circumstances, blood begins to clot within 3-8 minutes after leaving blood vessels – this is a crucial component of the body’s hemostatic mechanism. However, for certain laboratory tests, we need blood to remain in liquid form, which requires anticoagulants to prevent the clotting process.
Second is the continuation of cellular metabolism. Even outside the body, blood cells continue their metabolic activities. For instance, red blood cells continuously consume glucose through glycolysis, causing blood glucose levels to gradually decrease; white blood cells continue consuming oxygen, affecting blood gas analysis results.
Third is the variation in enzyme activities. Many laboratory tests depend on measuring enzyme activities in blood, and these activities change with time, temperature, pH, and other conditions.
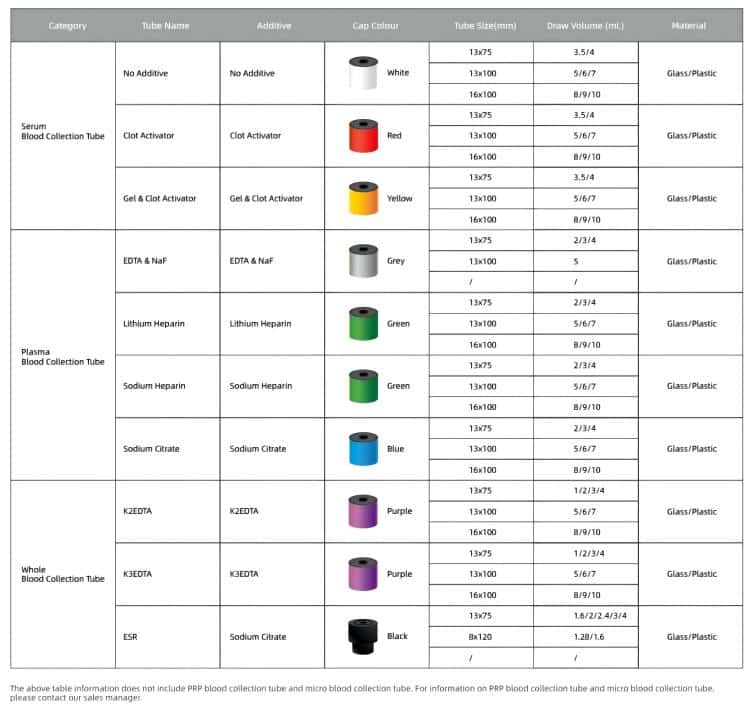
Mechanisms of Anticoagulant Action
Different anticoagulants prevent blood clotting through various mechanisms, determining their suitability for different laboratory tests.
Chelating anticoagulants like EDTA and sodium citrate work by binding calcium ions in blood to prevent coagulation. Calcium ions are essential cofactors in multiple steps of the coagulation process, and once chelated, the entire coagulation cascade cannot proceed. However, this chelating action also affects certain enzyme reactions that require calcium ions, making these tubes unsuitable for certain biochemical tests.
Enzyme-inhibiting anticoagulants like heparin work by activating antithrombin III to inhibit thrombin activity, thereby preventing the conversion of fibrinogen to fibrin. Heparin’s advantage is that it doesn’t significantly alter ion concentrations in blood, making it suitable for most biochemical tests.
Metabolic inhibitors like sodium fluoride, while not strictly anticoagulants, can inhibit glycolysis, maintaining stable glucose concentrations. This is crucial for accurate glucose measurement, especially when sample transport times are extended.
Ensuring Test Result Accuracy
Incorrect tube selection can lead to various problems. The most common is test result deviation. For example, using an EDTA tube for calcium measurement would yield significantly low results due to EDTA’s calcium-chelating properties, potentially leading to a misdiagnosis of hypocalcemia.
Hemolysis is another frequent issue. Improper tube selection or handling can cause red blood cell rupture, releasing intracellular contents that interfere with multiple tests. For instance, intracellular potassium concentrations are much higher than plasma levels, so hemolysis leads to falsely elevated potassium results.
Sample clotting is also problematic. If a test requiring anticoagulation is mistakenly collected in a non-anticoagulated tube, the sample will clot and become unusable, necessitating recollection.
Detailed Classification and Characteristics of Common Blood Collection Tubes
1. Purple/Lavender Tubes (EDTA Anticoagulant) – The Gold Standard for Hematology
Purple tubes are among the most frequently used blood collection tubes in clinical practice, with their inner walls coated with ethylenediaminetetraacetic acid (EDTA). EDTA is a powerful chelating agent that rapidly and irreversibly binds calcium and magnesium ions in blood.
EDTA’s Anticoagulation Mechanism in Detail
The EDTA molecule contains four carboxyl groups and two amino groups, enabling it to form six-coordinate chelate complexes. When blood enters an EDTA tube, EDTA molecules rapidly capture free calcium ions in the blood, forming stable EDTA-Ca2+ complexes. Since calcium ions are key cofactors in multiple coagulation steps, once chelated, coagulation reactions cannot proceed.
This chelating action not only prevents coagulation but also excellently preserves cell morphology. Compared to other anticoagulants, EDTA has minimal impact on cell membranes, maximally maintaining the original morphology and size of red blood cells, white blood cells, and platelets – crucial for accurate cell counts and morphological examinations.
Specific Applications
Complete blood count (CBC) is the primary application for purple tubes. Modern automated hematology analyzers can accurately measure red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). These parameters are vital for diagnosing various anemias, hematologic disorders, and infectious diseases.
White blood cell differential counting also requires EDTA tubes. Modern analyzers not only accurately count total white blood cells but can also perform five-part differentials: neutrophils, lymphocytes, monocytes, eosinophils, and basophils. This is valuable for diagnosing blood disorders, immune system diseases, and monitoring chemotherapy effects.
Platelet counting similarly requires EDTA tubes. Platelet count and function testing are crucial for diagnosing bleeding disorders, preoperative assessment, and anticoagulation therapy monitoring.
For blood typing, EDTA tubes are the standard choice. ABO and Rh blood type determination requires maintaining intact red blood cell morphology, which EDTA’s gentle anticoagulation can provide.
Critical Usage Notes
While EDTA tube usage seems simple, several key points require attention. First is the importance of mixing. After blood collection, tubes must be immediately and gently inverted 8-10 times. Insufficient mixing may cause localized clotting, affecting test accuracy. However, excessive mixing should be avoided as vigorous agitation can cause hemolysis.
Second is blood volume control. The anticoagulant amount in EDTA tubes is calculated for standard blood volumes. If insufficient blood is collected, excess anticoagulant may cause cell shrinkage; if too much blood is collected, insufficient anticoagulant may lead to localized clotting.
Finally, timing requirements matter. Although EDTA effectively preserves cell morphology, cells still undergo changes over time. Generally, CBC testing should be completed within 4 hours of collection to ensure result accuracy.
2. Red Tubes (No Additive/Clot Activator) – The Standard for Serum Testing
Red tubes are the most traditional blood collection tubes, containing no anticoagulants or other additives, also called plain tubes or serum tubes. This seemingly “empty” design actually reflects the special requirements of serum testing.
The Biological Basis of Serum Formation
When blood enters a red tube, natural coagulation occurs. This process can be divided into three phases: First is platelet aggregation, where platelets rapidly adhere to tube walls and aggregate with each other, forming primary hemostatic plugs; next is coagulation factor activation, where cascading coagulation factors are sequentially activated; finally is fibrin formation, where thrombin converts fibrinogen to fibrin, creating stable blood clots.
The entire coagulation process typically takes 30-60 minutes. During this process, blood cells and platelets become trapped in the fibrin network while serum separates out. The main difference between serum and plasma is that serum lacks fibrinogen and most coagulation factors while retaining other plasma proteins and biochemical components.
Wide Range of Applications
Serum biochemistry is the primary application for red tubes. Liver function tests include alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, direct bilirubin, total protein, and albumin measurements. These parameters are vital for diagnosing hepatitis, cirrhosis, liver cancer, and other hepatic diseases.
Kidney function tests also require red tubes, including blood urea nitrogen (BUN), creatinine, and uric acid. These markers reflect renal filtration and excretion functions, valuable for early chronic kidney disease diagnosis and monitoring.
Lipid panels must use serum, including total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). These markers are crucial for cardiovascular risk assessment and treatment monitoring.
In immunological testing, various antibody and antigen assays require serum. Examples include hepatitis B panel, hepatitis C antibodies, HIV antibodies, syphilis antibodies for infectious disease markers; rheumatoid factor and antinuclear antibodies for autoimmune disorders; and tumor markers like AFP, CEA, and PSA.
Endocrine hormone testing is another important application. Thyroid function tests (TSH, T3, T4), reproductive hormones (FSH, LH, E2, testosterone), insulin, and C-peptide all require serum.
Operational Key Points and Precautions
Red tube usage is relatively straightforward but has key considerations. After blood collection, immediate mixing is unnecessary – blood should be allowed to clot naturally. At room temperature, blood typically completes clotting within 30 minutes.
After clotting, centrifugation for separation is required. Standard centrifugation conditions are 3000 rpm for 10 minutes. Post-centrifugation, serum separates as a pale yellow, clear liquid on top; the bottom layer is the blood clot, appearing dark red.
During serum separation, careful technique prevents aspirating blood clots. If blood cells contaminate serum, test results may be affected. Separated serum should be clear and transparent; if hemolyzed (red) or lipemic (milky white), recollection is necessary.
For certain special tests, patient preparation is important. For example, lipid panels typically require 12-hour fasting; liver function tests should avoid alcohol consumption beforehand; certain hormone tests require collection at specific times.
3. Gold/Yellow Tubes (Serum Separator Tubes) – Optimized Choice for Modern Serum Testing
Gold tubes are improved versions of traditional red tubes, with a special inert separator gel added at the tube bottom. This seemingly simple improvement greatly enhances serum testing efficiency and quality.
Separator Gel Working Principle
Separator gel is an inert substance with specific gravity between serum and blood clots, typically made from polyester compounds. At room temperature, the gel remains solid at the tube bottom; during centrifugation, centrifugal forces cause the gel to become semi-fluid and, due to its specific gravity, automatically migrate between serum and blood clots, forming a physical barrier.
This barrier serves crucial functions. First, it completely separates serum from blood clots, preventing cellular components from leaching into serum and ensuring serum purity. Second, it creates a sealed layer so that even during transport or storage agitation, serum and blood clots won’t remix.
Technical Advantages Analysis
Compared to traditional red tubes, gold tubes offer multiple technical advantages. The most significant is reduced hemolysis. Hemolysis is the most common interference factor in serum testing, affecting multiple test accuracies. Separator gel presence better protects red blood cells, reducing mechanical hemolysis occurrence.
Second is improved separation efficiency. Traditional red tubes may not have clear serum-clot interfaces after centrifugation, making serum separation prone to cellular contamination. Separator tubes create distinct interfaces, making serum separation easier and more complete.
The third advantage is extended storage time. Due to separator gel protection, separated serum can be stored longer at room temperature without deterioration. This is particularly meaningful for specimens requiring batch testing or long-distance transport.
Clinical Application Features
Gold tubes are especially suitable for emergency testing. In emergency departments, time is life, and rapid, accurate test results are crucial for patient care. Separator tubes can provide high-quality serum samples in shorter timeframes, meeting emergency testing needs.
For batch serum testing, gold tubes also show clear advantages. In health screening centers or large hospital laboratories processing hundreds or thousands of serum specimens daily, separator tubes significantly improve work efficiency and reduce manual serum separation workload.
For specimen transport, gold tubes offer advantages. For specimens requiring external laboratory testing, separator gel protection ensures specimens maintain good condition during transport.

4. Blue Tubes (Sodium Citrate Anticoagulant) – Dedicated Tubes for Coagulation Studies
Blue tubes hold the most specialized position among all collection tubes because they’re dedicated to coagulation studies. Accurate coagulation testing is crucial for diagnosing bleeding disorders, monitoring anticoagulation therapy, and preoperative assessment.
Sodium Citrate’s Anticoagulation Mechanism
Blue tubes contain 3.2% or 3.8% sodium citrate solution. Sodium citrate is a chelating agent whose anticoagulation principle involves chelating calcium ions in blood. Unlike EDTA, sodium citrate chelation is reversible – a characteristic crucial for coagulation testing.
During coagulation studies, laboratories add excess calcium ions to plasma, releasing chelated calcium and restoring blood’s coagulation ability. Specific activators then initiate coagulation processes while measuring clotting times. This “inhibit then activate” method standardizes testing conditions, improving result accuracy and reproducibility.
Detailed Explanation of Major Test Items
Prothrombin Time (PT) is a classic indicator evaluating the extrinsic coagulation pathway. PT measures time from tissue factor activation to fibrin formation, primarily reflecting factors VII, X, V, II, and fibrinogen function. PT prolongation commonly occurs in vitamin K deficiency, liver dysfunction, and warfarin anticoagulation therapy.
Activated Partial Thromboplastin Time (aPTT) evaluates the intrinsic coagulation pathway. aPTT measures time from contact activation to fibrin formation, primarily reflecting factors XII, XI, IX, VIII, X, V, II, and fibrinogen function. aPTT prolongation commonly occurs in hemophilia, heparin anticoagulation therapy, and acquired coagulation factor inhibitors.
Thrombin Time (TT) primarily evaluates fibrinogen conversion to fibrin. TT prolongation commonly occurs in fibrinogen deficiency or abnormalities, heparin contamination, and increased fibrin degradation products.
Fibrinogen (Fbg) is the final substrate in coagulation and an acute-phase reactant protein. Fibrinogen level measurement is important for assessing both coagulation function and inflammatory status.
Strict Operational Requirements
Blue tube usage requires stricter protocols than other tubes. First, blood-to-anticoagulant ratio must be strictly maintained at 9:1. This ratio has been validated through extensive experimentation as optimal; any deviation can affect test results. Insufficient blood volume with excess anticoagulant causes prolonged clotting times; excessive blood volume with insufficient anticoagulant may cause localized clotting.
Second, tubes must be filled completely. Blue tubes typically have fill lines indicating required blood volumes. Only when blood reaches standards can proper blood-to-anticoagulant ratios be ensured.
Mixing is also critical. After collection, tubes must be immediately and gently inverted 5-8 times, ensuring thorough blood-anticoagulant mixing. However, excessive mixing should be avoided as vigorous agitation may activate platelets, affecting test results.
Timing requirements are equally strict. Coagulation studies must be completed within 2 hours of collection, preferably within 1 hour. Delayed testing causes decreased coagulation factor activity, affecting result accuracy.
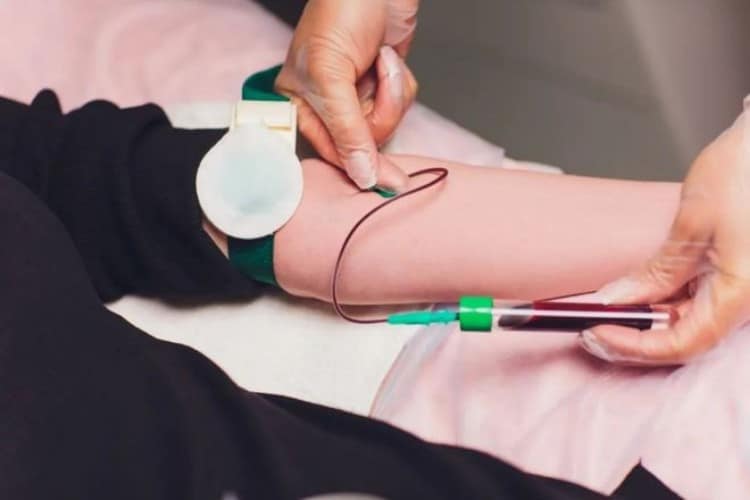
5. Green Tubes (Heparin Anticoagulant) – Ideal Choice for Emergency Biochemistry
Green tubes contain heparin as anticoagulant and are among the most important collection tubes in emergency medicine and critical care. Heparin’s unique anticoagulation mechanism makes it the preferred choice for emergency biochemistry and blood gas analysis.
Detailed Heparin Anticoagulation Mechanism
Heparin is a natural anticoagulant widely present in mast cells and basophils. Its anticoagulation mechanism differs completely from other anticoagulants: heparin works by activating antithrombin III (AT-III).
Normally, AT-III’s thrombin inhibition is relatively slow. However, when heparin binds to AT-III, it causes conformational changes in the AT-III molecule, enhancing its thrombin inhibition capability by over 1000-fold. The heparin-AT-III complex not only rapidly neutralizes thrombin but also inhibits multiple coagulation factors including factors Xa, IXa, XIa, and XIIa.
This anticoagulation mechanism’s advantage is that it doesn’t significantly alter blood electrolyte concentrations, particularly without chelating calcium and magnesium ions. This makes heparin tubes very suitable for various biochemical tests, especially those requiring accurate ion concentration measurements.
Differences Between Sodium Heparin and Lithium Heparin
Clinical heparin tubes are mainly two types: sodium heparin and lithium heparin tubes. Both have essentially identical anticoagulation effects but differ in certain test applications.
Sodium heparin tubes are suitable for most biochemical tests but not for sodium ion measurement since sodium heparin contains sodium ions that would interfere with results. Additionally, sodium heparin tubes aren’t suitable for certain enzyme activity measurements as sodium ions may affect enzyme activities.
Lithium heparin tubes have broader applications, suitable for most biochemical tests including sodium ion measurement. Lithium ions have minimal effects on most enzyme activities, making lithium heparin tubes considered more ideal for emergency biochemistry.
Special Advantages in Emergency Applications
In emergency medicine, time is often more precious than gold. Green tubes’ greatest advantage is rapid sample availability. Unlike red tubes requiring clotting and centrifugation, green tubes can be tested immediately after collection and mixing – crucial for critically ill patients requiring rapid diagnosis.
Blood gas analysis is a classic application for green tubes. Blood gas analysis measures blood pH, oxygen tension, carbon dioxide tension, and bicarbonate concentrations – parameters vital for assessing acid-base balance and oxygenation status. Blood gas analysis typically requires immediate testing after collection, and heparin’s rapid anticoagulation meets this requirement.
In emergency biochemistry, green tubes show similar advantages. For patients requiring urgent surgery, preoperative biochemistry (electrolytes, liver/kidney function) must be completed in minimal time. Green tubes eliminate clotting wait times, providing rapid test results.
Usage Precautions
While green tubes offer numerous advantages, usage requires attention to certain issues. First is mixing importance. After collection, tubes must be immediately and gently inverted 5-8 times, ensuring thorough heparin-blood mixing. Insufficient mixing may cause localized clotting, affecting test results.
Second is heparin dosage control. Excessive heparin may affect certain enzyme activity measurements; insufficient amounts may cause inadequate anticoagulation. Standard heparin tubes undergo precise dosage control, so strict adherence to blood volume requirements is essential.
Finally, heparin tubes aren’t suitable for certain special tests. For example, heparin interferes with some immunological assays, making it unsuitable for complement testing; heparin may also affect certain enzyme-linked immunosorbent assay (ELISA) results.

6. Gray Tubes (Sodium Fluoride/Potassium Oxalate) – Specialized Tubes for Glucose Testing
Gray tubes are highly specialized collection tubes designed specifically for accurate glucose and lactate measurement. Their unique dual-additive system represents modern laboratory specimen quality control pursuit of precise testing.
Dual-Additive System Design Rationale
Gray tubes contain two additives: sodium fluoride and potassium oxalate. This dual-additive system design reflects profound biochemical principles.
Potassium oxalate serves as anticoagulant, chelating calcium ions to prevent blood clotting. Potassium oxalate was chosen over other anticoagulants because it has minimal impact on glycolysis while maintaining blood’s liquid state without interfering with glucose measurement.
Sodium fluoride’s role is more specialized as a potent glycolysis inhibitor. Sodium fluoride irreversibly inhibits enolase activity, a key enzyme in glycolysis. By inhibiting this enzyme, sodium fluoride effectively blocks glycolysis, maintaining stable blood glucose concentrations.
Impact of Glycolysis on Glucose Testing
Understanding glycolysis is crucial for recognizing gray tubes’ importance. Normally, after blood leaves circulation, red blood cells, white blood cells, and platelets continue metabolic activities. Red blood cells primarily obtain energy through glycolysis, continuously consuming blood glucose.
At room temperature, blood glucose decreases at 5-7 mg/dL per hour. This means a sample with 100 mg/dL glucose might decrease to 85-90 mg/dL after 2 hours at room temperature – a change sufficient to affect clinical diagnosis.
For diabetic patients’ glucose monitoring, this change is even more significant. Diabetes diagnostic criteria are strict: fasting glucose ≥126 mg/dL or random glucose ≥200 mg/dL. If glycolysis causes falsely low glucose values, diabetes might be missed or glucose control incorrectly assessed.
Special Test Applications
Beyond glucose testing, gray tubes serve important roles in several other specialized tests.
Lactate measurement is another important gray tube application. Lactate is a product of anaerobic cellular metabolism, and its blood concentration measurement is important for assessing tissue hypoxia, shock severity, and certain metabolic diseases. Like glucose, blood continues producing lactate after collection, causing elevated measured values. Sodium fluoride prevents this in vitro lactate generation by inhibiting glycolysis, ensuring result accuracy.
In certain special metabolic studies, gray tubes are used for other sugar measurements like fructose and galactose. These sugars are similarly affected by glycolysis, and sodium fluoride maintains their concentration stability.
Special Clinical Significance
Gray tubes hold special positions in diabetes management. Diabetes is a chronic metabolic disease requiring long-term glucose monitoring. Accurate glucose testing not only relates to disease diagnosis but directly impacts treatment plan development and adjustment.
For glucose tolerance tests (OGTT), gray tubes are indispensable. OGTT requires multiple blood glucose measurements at different time points after glucose ingestion, with the entire process potentially lasting 2-3 hours. Without gray tubes, later-collected samples might experience significant glucose decreases during testing delays, affecting test result accuracy.
In emergency medicine, rapid and accurate glucose and lactate testing is vital for differential diagnosis in comatose patients. Hypoglycemic coma, diabetic ketoacidosis, and hyperosmolar coma all require accurate glucose testing for diagnosis. Lactate level measurement helps assess shock severity and prognosis.
7. Black Tubes (High-Concentration Sodium Citrate) – Specialized Tubes for ESR Testing
Black tubes are relatively specialized collection tubes mainly used for erythrocyte sedimentation rate (ESR) testing. Though used less frequently than other tubes, they play irreplaceable roles in diagnosing and monitoring certain diseases.
Biological Basis of ESR Testing
ESR refers to the sedimentation rate of red blood cells in plasma under specific conditions. This seemingly simple physical phenomenon actually reflects complex biological processes.
Normally, red blood cell surfaces carry negative charges, creating electrostatic repulsion between cells, causing them to remain dispersed in plasma. However, when certain plasma proteins (particularly fibrinogen and immunoglobulins) increase, these proteins adsorb onto red blood cell surfaces, reducing intercellular electrostatic repulsion and facilitating red blood cell aggregation into chains, forming “rouleaux formation.”
Aggregated red blood cells, due to increased volume and relatively higher density, settle faster under gravity, causing accelerated ESR. Therefore, ESR speed indirectly reflects plasma acute-phase protein levels, serving as a non-specific inflammation marker.
Special Requirements for High-Concentration Sodium Citrate
Black tubes contain 3.8% sodium citrate, higher concentration than blue tubes’ 3.2%. This high-concentration design has special considerations.
ESR testing requires specialized ESR tubes (like Westergren tubes), diluting anticoagulated blood to specific ratios before testing. International standards require 4:1 blood-to-sodium citrate ratios, providing the most stable testing conditions and reducing various interference factors.
High-concentration sodium citrate also more effectively chelates calcium ions, ensuring blood remains liquid during extended periods – important for ESR testing standardization since ESR testing typically requires 1-hour observation periods, and any microscopic clotting could affect result accuracy.
Clinical Application Value
Though ESR is a non-specific indicator, it retains important clinical value. In rheumatic immunological disease diagnosis, ESR often serves as an important reference indicator. For example, rheumatoid arthritis, systemic lupus erythematosus, and vasculitis during active phases often show significantly accelerated ESR.
In infectious disease diagnosis and monitoring, ESR also plays important roles. During acute infections, due to massive acute-phase protein production, ESR markedly accelerates. By monitoring ESR changes, infection severity and treatment effectiveness can be assessed.
Certain malignancies may also cause accelerated ESR, particularly hematologic malignancies like multiple myeloma and lymphomas. Though ESR cannot serve as a specific tumor diagnostic indicator, it can be used as a reference for disease monitoring.
Testing Methods and Quality Control
Traditional Westergren method is the classic ESR testing approach. Anticoagulated blood is mixed with sodium citrate at 4:1 ratios, thoroughly mixed, then drawn into specialized ESR tubes and placed vertically for 1 hour before reading red blood cell descent in millimeters.
Modern laboratories often use automated ESR analyzers that can complete testing in shorter timeframes while improving result accuracy and reproducibility. Regardless of method used, strict quality control is required, including temperature control, tube verticality, and reading accuracy.
Key Points for Blood Collection Tube Usage
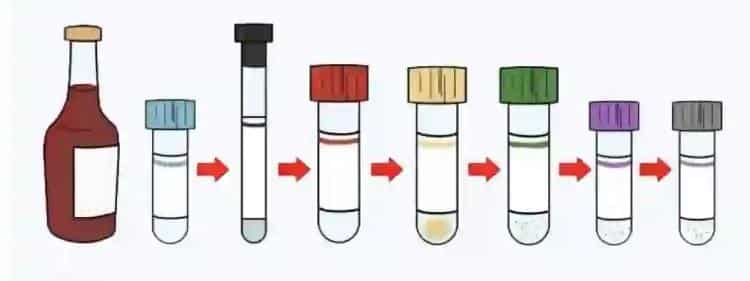
Correct Collection Order and Scientific Rationale
Collection order determination isn’t arbitrary but based on strict scientific principles and extensive experimental validation. Incorrect collection order can cause cross-contamination between different tubes, affecting test result accuracy.
Cross-Contamination Mechanisms
When using the same needle to consecutively collect multiple tubes, additives from previous tubes may carry through the needle to subsequent tubes – this phenomenon is called cross-contamination. Even trace contamination can significantly impact certain sensitive tests.
For example, if EDTA tubes are collected before coagulation tubes, trace EDTA might contaminate sodium citrate tubes. Since EDTA’s chelating capacity far exceeds sodium citrate, even minute EDTA amounts can cause abnormally prolonged clotting times.
Standard Collection Order Development
The International Federation of Clinical Chemistry (IFCC) and Clinical and Laboratory Standards Institute (CLSI), after extensive research, established standard collection orders:
- Blood culture bottles (if needed): Blood cultures require extreme sterility and must be collected first to avoid any potential contamination.
- Red tubes (serum tubes): Additive-free tubes rank second to avoid contamination from other additives.
- Blue tubes (coagulation): Sodium citrate is relatively sensitive to cross-contamination, so earlier positioning reduces contamination risk.
- Green tubes (heparin): Heparin tubes occupy middle positions, avoiding strong chelator contamination while not significantly affecting subsequent tubes.
- Purple tubes (EDTA): EDTA is a strong chelator, positioned later to avoid contaminating other tubes.
- Gray tubes (glucose): Sodium fluoride has toxicity, positioned last to avoid contaminating other samples.
Special Situation Handling
In certain special situations, collection order may need adjustment. For example, if patients only need CBC testing, EDTA tubes can be used directly without considering order issues.
If multiple special tests require simultaneous blood collection, order should be arranged according to test importance and sample stability. Generally, more important and urgent tests should be prioritized; less stable samples prone to changes should also be prioritized.
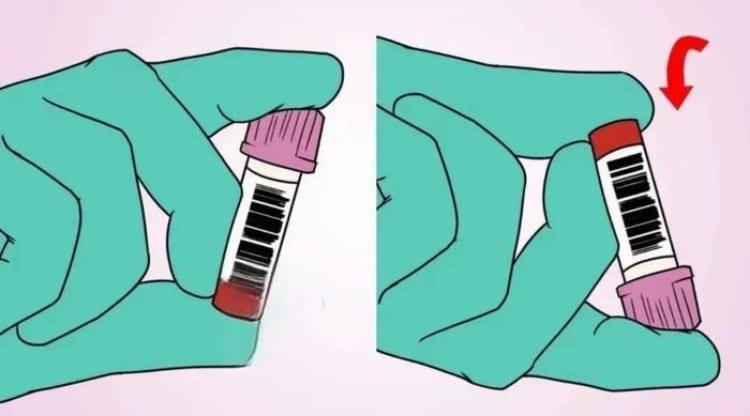
Mixing Methods and Frequency Scientific Rationale
Mixing is a critical step ensuring thorough anticoagulant-blood combination, but different tubes require different mixing methods and frequencies.
Biological Significance of Mixing
When blood enters tubes containing anticoagulants, anticoagulants need sufficient contact with relevant blood components to function effectively. Insufficient mixing may cause localized blood clotting, affecting test results; excessive mixing may cause cellular damage or protein denaturation.
Different Tube Mixing Requirements
Purple tubes (EDTA) require gentle inversion 8-10 times. EDTA needs thorough contact with calcium ions in blood, while EDTA’s cell membrane protection requires appropriate mixing. Too few inversions may cause localized clotting; too many may cause hemolysis.
Blue tubes (coagulation) require gentle inversion 5-8 times. Sodium citrate’s anticoagulation is relatively mild, and excessive mixing may activate platelets, affecting coagulation testing. Therefore, mixing frequency is relatively fewer.
Green tubes (heparin) also require gentle inversion 5-8 times. Heparin’s anticoagulation is rapid, but thorough blood mixing must be ensured.
Gray tubes (glucose) require gentle inversion 8-10 times. Sodium fluoride needs thorough dispersion throughout blood to effectively inhibit glycolysis, requiring more mixing.
Red tubes don’t require mixing since their purpose is natural blood clotting.
Mixing Technique Requirements
Proper mixing should involve gentle inversion rather than vigorous shaking. Inversion should allow blood to flow from one tube end to the other, ensuring thorough blood-tube wall contact. The entire motion should be smooth, avoiding bubble formation or mechanical damage.
Mixing timing is also important. Mixing should occur immediately after blood collection, not waiting until all collection is complete for batch mixing. Delayed mixing may cause localized clotting, affecting anticoagulation effectiveness.
Common Error Analysis and Prevention Measures
Error 1: Incorrect Tube Selection
This is the most common and serious error. Wrong tube selection can cause completely incorrect test results, affecting clinical diagnosis and treatment.
Prevention Measures:
- Establish standardized tube selection guidelines clearly specifying corresponding tube types for different tests
- Strengthen healthcare personnel training ensuring everyone clearly understands various tube applications
- Place quick reference cards on collection carts or trays showing corresponding tubes for common tests
- Establish double-checking mechanisms, especially for special tests
Case Analysis: A hospital once experienced an incident using EDTA tubes for coagulation studies. Since EDTA chelates calcium ions, it caused infinitely prolonged clotting times, with the laboratory reporting “unable to clot.” The patient was mistakenly thought to have severe coagulation dysfunction, nearly canceling scheduled surgery. Later discovery revealed incorrect tube selection, and recollection with blue tubes showed completely normal coagulation function.
Error 2: Insufficient or Excessive Mixing
Insufficient mixing is another common problem, particularly when staff is busy and might overlook this step.
Prevention Measures:
- Develop standardized mixing protocols clearly specifying mixing frequency and methods for each tube type
- Use timers or counters to assist, ensuring accurate mixing frequency
- Regular training and assessment to reinforce correct mixing techniques
- Label tubes with mixing requirements to remind operators
Actual Impact: The most common consequence of insufficient mixing is localized clotting. Microscopic examination can reveal small clots in blood smears, affecting cell count accuracy. Severe cases may cause instrument blockages, requiring recollection.
Error 3: Insufficient or Excessive Blood Volume
Blood volume control is crucial for test result accuracy, especially for anticoagulated tubes.
Prevention Measures:
- Use standardized vacuum collection systems ensuring consistent blood volumes each time
- Clearly mark standard blood volumes on collection tubes
- Train healthcare personnel to recognize adequate blood volumes
- Recollect samples with insufficient blood volumes
Scientific Rationale: Using blue tubes as an example, if blood volume is only half the standard amount, the anticoagulant-to-blood ratio becomes 1:4.5 instead of the standard 1:9, causing approximately 10-15% prolonged clotting times, potentially affecting clinical judgment.
Error 4: Improper Post-Collection Handling
Post-collection handling is equally important, including specimen identification, storage, and transport.
Prevention Measures:
- Establish complete specimen management processes with clear requirements for every step from collection to testing
- Use barcode systems for specimen identification, reducing human errors
- Develop corresponding storage and transport conditions based on different test requirements
- Establish specimen quality assessment systems for timely identification and handling of unqualified specimens
Temperature Control: Different tests have varying storage temperature requirements. CBC can be stored at room temperature for 4 hours; coagulation studies need completion within 2 hours at room temperature; certain special tests may require refrigeration or freezing.
Comprehensive Quality Control System
Pre-Collection Preparation
Standardized Patient Preparation
Patient preparation directly affects test result accuracy. Different tests have varying patient preparation requirements, all based on profound physiological and biochemical principles.
Fasting requirements are the most common patient preparation. Lipid panels typically require 12-hour fasting because postprandial blood triglycerides significantly elevate, potentially lasting 8-12 hours. Non-fasting testing might cause overestimated lipid levels, affecting cardiovascular risk assessment.
Glucose testing has slightly different fasting requirements. Fasting glucose requires 8-12 hours fasting; insufficient time may be affected by previous meals; excessive time may cause abnormally low glucose due to starvation or stress-induced elevation.
Certain hormone tests have special timing requirements. For example, cortisol has distinct circadian rhythms with 8-10 AM being optimal testing time; growth hormone secretes most during sleep, requiring specific timing for collection.
Equipment and Supply Quality Control
Collection equipment quality directly impacts collection success rates and sample quality. Needle selection should be based on patient vascular conditions and blood volume requirements. Needles too fine may cause collection difficulties and hemolysis; needles too large may increase patient discomfort and vascular damage.
Collection tube quality control is equally important. Tubes must be checked for expiration dates, vacuum integrity, and additive completeness. Expired tubes may lose vacuum or have ineffective additives; insufficient vacuum may cause collection difficulties or inadequate blood volumes.
Environmental Condition Control
Collection environments affect both patient physiological status and sample quality. Excessive temperatures may cause patient sweating and vasodilation, affecting certain tests; low temperatures may cause vasoconstriction, increasing collection difficulty.
Lighting conditions also require consideration. Certain tests like bilirubin are light-sensitive, requiring light-protected storage. Collection room lighting should be gentle yet adequate for operators to observe vessels and perform procedures.
Quality Control During Collection
Standardized Operating Procedures
Developing detailed Standard Operating Procedures (SOPs) is the foundation of quality control. SOPs should include specific requirements for patient identity verification, collection site selection, disinfection procedures, collection techniques, and sample handling.
Patient identity verification is the first line of defense against specimen errors. At least two methods should verify patient identity, such as name and birth date, or name and medical record number. Hospitals with capacity should use wristband scanning systems for further accuracy improvement.
Collection site selection requires comprehensive consideration of multiple factors. The median cubital vein in the antecubital fossa is preferred, but if vascular conditions are poor, cephalic or basilic veins may be chosen. Sites with inflammation, sclerosis, or fistulas should be avoided.
Standardized Collection Techniques
Proper collection techniques reduce patient discomfort, improve collection success rates, and ensure sample quality. Tourniquet application shouldn’t exceed 1 minute; prolonged application may cause hemoconcentration, affecting test results.
Needle insertion angle and depth should be adjusted based on vessel position and depth. Generally, insertion angles are 15-30 degrees; excessive angles may penetrate vessels, while insufficient angles may fail to enter vessel lumens.
Collection speed also requires control. Excessive speed may cause hemolysis, especially with fine needles; insufficient speed may cause blood clotting, affecting subsequent tube collection.
Real-time Quality Monitoring
Sample quality requires real-time observation during collection. Normal blood should be bright red with good fluidity. If hemolysis occurs (blood appears dark red or foamy), recollection should be considered.
Collection volume control requires observing vacuum tube pressure changes. Normally, blood should flow automatically into collection tubes; if manual aspiration is needed, problems may exist.
Post-Collection Sample Handling
Timely Correct Identification
Sample identification is crucial for preventing specimen errors. Identification should occur in front of patients, including patient name, collection date, collection time, and collector name. When possible, barcode identification systems should be used.
Label positioning also has requirements. Labels should be affixed to tube bodies, not caps, as caps may be lost or replaced. Labels should adhere firmly, avoiding detachment during transport.
Appropriate Storage and Transport
Different tests have varying storage and transport requirements. CBC samples can be stored at room temperature for 4 hours but should avoid direct sunlight and high temperatures.
Coagulation samples have stricter temperature and time requirements. They should be stored at room temperature, avoiding refrigeration as low temperatures may affect coagulation factor activity. Transport time shouldn’t exceed 2 hours.
Certain special tests require special storage conditions. Vitamin tests need light protection; certain enzyme activity tests need low-temperature storage; certain hormone tests require immediate serum separation and freezing.
Quality Assessment and Feedback
Establish comprehensive quality assessment systems for continuous collection quality monitoring. Key indicators include hemolysis rates, specimen rejection rates, and recollection rates.
Hemolysis rate is an important indicator for assessing collection techniques. Normally, hemolysis rates should be controlled below 2%. If rates are excessive, cause analysis is needed – possibly collection technique issues or equipment problems.
Specimen rejection rates reflect overall collection process quality. Rejection causes may include insufficient blood volume, clotting, or contamination. Each rejected specimen requires cause analysis and corresponding improvement measures.
Establish feedback mechanisms to promptly relay laboratory-identified problems to collection personnel. Regular quality analysis meetings should discuss existing problems and improvement measures.
Case Studies: In-Depth Analysis
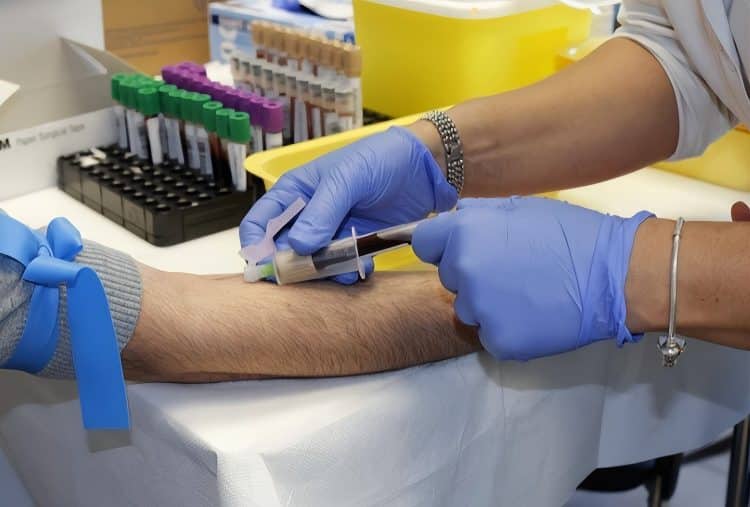
Case 1: Emergency Critical Care Collection Challenges
Case Background
A tertiary hospital emergency department admitted a 68-year-old male presenting with “chest pain for 2 hours.” The patient had histories of hypertension and diabetes, with long-term aspirin antiplatelet therapy. Upon admission, the patient was confused with blood pressure 80/50 mmHg, heart rate 120 bpm, and dyspnea.
The emergency physician preliminarily diagnosed acute myocardial infarction with cardiogenic shock, requiring urgent multiple tests: cardiac enzymes, troponins, CBC, coagulation studies, blood gases, electrolytes, and liver/kidney function.
Collection Strategy Development
Facing such critical patients, collection strategy development required comprehensive consideration of multiple factors:
- Time urgency: Patients in shock require every minute to be precious; all necessary tests must be completed in minimal time.
- Vascular conditions: Shock patients, due to hypovolemia and vasoconstriction, have poor peripheral vessel filling, making collection difficult.
- Test priority: Clinical needs must determine test priority order.
- Patient safety: While ensuring test needs, minimize patient trauma.
Specific Implementation Process
The charge nurse, with extensive experience, decided on a central venous collection strategy. Since the patient required central venous access for resuscitation, this opportunity could simultaneously accomplish collection.
Collection order arrangement:
- First collect blood cultures (suspecting possible septic shock)
- Red tubes ×2 (for cardiac enzymes, troponins, biochemistry)
- Blue tube ×1 (coagulation function, assess bleeding risk)
- Green tube ×1 (blood gases, assess acid-base balance and oxygenation)
- Purple tube ×1 (CBC, understand infection status)
Challenges Encountered and Solutions
Challenge 1: Poor vascular filling Due to patient shock, peripheral vessels were poorly filled, making routine antecubital collection nearly impossible.
Solution: Internal jugular vein central line placement met both therapeutic needs and solved collection difficulties. This “two birds, one stone” strategy is frequently used in emergency medicine.
Challenge 2: Slow blood flow Due to low blood pressure and slow blood flow, blood entered collection tubes slowly, increasing clotting risk.
Solution: Used larger gauge needles (18G) and strictly followed collection order, immediately mixing each completed tube appropriately.
Challenge 3: Sample volume balance Critically ill patients shouldn’t have excessive blood drawn, but numerous tests required reasonable blood volume distribution.
Solution: Communicated with laboratory – certain tests could share samples. For example, red tube serum could be used for both cardiac enzymes and biochemistry; optimized test items, deferring non-urgent tests.
Results and Reflection
Through careful strategy development and implementation, all urgently needed tests were successfully completed. Results showed: troponin I significantly elevated (>50 ng/mL), confirming acute ST-elevation myocardial infarction; blood gases showed metabolic acidosis; coagulation function was essentially normal; CBC indicated elevated white blood cells.
Based on these results, physicians rapidly developed treatment plans: emergency PCI (percutaneous coronary intervention), anticoagulation therapy, inotropic support, and diuretics. The patient was ultimately successfully resuscitated.
Key success factors analysis:
- Adequate contingency preparation: The charge nurse’s deep understanding of various tube characteristics enabled rapid development of reasonable collection strategies.
- Team collaboration: Close cooperation between physicians, nurses, and laboratory ensured efficient process execution.
- Technical proficiency: Collection technique proficiency directly affected success rates, especially under difficult conditions.
- Quality consciousness: Even in emergencies, strict adherence to standard collection procedures was maintained.
Case 2: Special Considerations for Pediatric Collection
Case Background
A children’s hospital admitted a 3-year-old girl presenting with “fever for 3 days, rash for 1 day.” The child had a temperature of 39.5°C with scattered petechiae throughout her body, causing parental anxiety. The pediatrician suspected acute leukemia or idiopathic thrombocytopenic purpura, requiring CBC and pre-bone marrow coagulation studies.
Special Challenges in Pediatric Collection
Pediatric collection is recognized as one of the most challenging nursing procedures, with main difficulties including:
- Poor vascular conditions: Children have small, fragile vessels prone to spasm and rupture.
- Poor cooperation: Children have severe fear, making procedural cooperation difficult.
- Blood volume limitations: Children have small blood volumes, preventing excessive collection.
- Parental emotions: Parents are often more anxious than patients, increasing operational difficulty.
Special Collection Strategy Design
Psychological preparation phase: Collection nurses first conducted thorough communication with the child and parents, explaining collection necessity and process to reduce fear. Cartoon stickers served as “bravery rewards,” establishing positive cooperation atmospheres.
Technical preparation phase: Selected finest collection needles (23G) to reduce pain and vascular damage. Prepared pediatric-specific small-volume collection tubes to avoid excessive blood collection.
Tube selection:
- Purple tube ×1 (0.5 mL, pediatric-specific): CBC
- Blue tube ×1 (1.8 mL): Coagulation assessment Considering the child’s condition and blood volume, temporarily performed only these two most important tests.
Optimized operational process: Selected the child’s hand dorsal vein as collection site – this location is relatively easy to stabilize with minimal impact on child activities. Employed “one-time success” strategy with experienced nurses performing procedures, avoiding repeated punctures causing child suffering.
Detailed Management During Implementation
Environmental control: Collection occurred in a dedicated pediatric treatment room with warm, child-friendly decorations and soft music, creating relaxing atmospheres.
Positioning arrangement: The child sat in her parent’s lap, providing security while facilitating parental assistance with positioning.
Attention diversion: During collection, nurses chatted with the child about favorite cartoons, effectively diverting attention and reducing fear.
Technical execution:
- Carefully observed vessel direction, selecting optimal puncture sites
- After disinfection, rapidly inserted needles with gentle but decisive movements
- Once blood began flowing, immediately comforted the child: “Look, the red blood flows like a little river”
- Strictly controlled collection time, avoiding prolonged tourniquet compression
- After completion, immediately applied pressure for hemostasis and affixed cute bandages
Result Analysis and Insights
Test results showed normal white blood cell counts but significantly decreased platelets (15×10^9/L) with normal coagulation function. Combined with clinical presentation, idiopathic thrombocytopenic purpura was diagnosed, and timely steroid treatment led to improvement.
Success factor summary:
- Adequate psychological preparation: Effective communication reduced child and parental anxiety
- Technical excellence: One-time successful collection avoided repeated puncture pain
- Humanized service: Considering issues from the child’s perspective, providing warm environments and thoughtful service
- Family involvement: Including parents in the treatment process increased child security
Experience summary:
- Pediatric collection success relies on combining technique with psychological care
- Environmental factors significantly impact children’s psychological states
- “Prevention is better than cure” applies to collection – one successful collection beats multiple failed attempts
Case 3: Blood Collection Management in Elderly Patients with Multiple Medications
Case Background
A geriatric department admitted an 85-year-old male complaining of “dyspnea on exertion for 1 month.” The patient had multiple chronic conditions including coronary artery disease, atrial fibrillation, hypertension, diabetes, and chronic kidney disease, with long-term multiple medications: warfarin, aspirin, metoprolol, ACE inhibitors, metformin, and insulin.
After admission, comprehensive assessment required multiple tests: CBC, comprehensive metabolic panel, coagulation studies, cardiac enzymes, BNP, blood gases, and HbA1c.
Complex Challenges in Elderly Patient Collection
Physiological challenges:
- Vascular sclerosis: Long-term hypertension and diabetes caused vessel wall thickening and reduced elasticity
- Thin skin: Elderly skin and subcutaneous tissue atrophy make vessels more vulnerable to injury
- Abnormal coagulation: Long-term warfarin and aspirin affect hemostatic function
Medication effects:
- Warfarin: Affects vitamin K-dependent coagulation factors, requiring special attention to coagulation testing
- Aspirin: Inhibits platelet aggregation, increasing bleeding risk
- Metformin: May affect vitamin B12 and folate metabolism, requiring related testing
Psychosocial factors:
- Cognitive decline: May affect cooperation
- Family anxiety: Children are particularly concerned about elderly examination processes
- Multiple medical experiences: May cause fear or resistance to collection
Comprehensive Collection Strategy Development
Risk assessment: First assessed patient bleeding risk. Since the patient took both warfarin and aspirin, bleeding risk was high, requiring special attention to hemostatic measures.
Tube selection strategy: Based on multiple test requirements, developed detailed tube lists:
- Purple tube ×1: CBC
- Red tubes ×3: Comprehensive metabolic panel, cardiac enzymes, BNP
- Blue tube ×1: Coagulation studies (particularly important for monitoring warfarin effects)
- Green tube ×1: Blood gases
- Gray tube ×1: HbA1c (important diabetes monitoring indicator)
Timing arrangement: Considering patient warfarin use, coagulation testing was crucial for assessing medication safety, scheduled for first morning collection to avoid other interfering factors.
Precise Implementation Management
Vascular assessment and selection: Due to severe vascular sclerosis, routine median cubital veins felt like “steel wires” with poor elasticity. After careful assessment, the charge nurse selected the relatively better basilic vein for puncture.
Special technique applications:
- Ultrasound-guided positioning: Used portable ultrasound devices to confirm vessel depth and direction
- Warm compresses: Applied warm compresses to puncture sites for 5 minutes before collection to promote vasodilation
- Special puncture techniques: Used “shallow angle, slow insertion” techniques to avoid penetrating sclerotic vessel walls
Anticoagulation management: Considering patient bleeding risk, developed special hemostatic protocols:
- Extended post-collection pressure time to 10 minutes (normal 3-5 minutes)
- Used elastic bandages for compression dressing for 30 minutes
- Checked puncture sites after 2 hours to confirm no bleeding
Test Result Interpretation and Clinical Significance
Test results showed multiple abnormalities:
- INR 3.2 (International Normalized Ratio, normal 0.8-1.2): Indicating excessive warfarin anticoagulation
- Hemoglobin 90 g/L: Mild anemia, possibly related to chronic kidney disease
- Creatinine 156 μmol/L: Mild kidney function decline
- BNP 800 pg/mL: Heart failure manifestation
- HbA1c 8.5%: Suboptimal glucose control
Based on these results, physicians promptly adjusted treatment plans:
- Reduced warfarin dosage to avoid bleeding risk
- Enhanced heart failure treatment, adjusted diuretic dosages
- Optimized glucose-lowering regimens, strengthened glucose monitoring
- Initiated further anemia investigation and treatment
Case Reflection and Experience Summary
Success factors:
- Comprehensive assessment: Fully considered patient disease status, medication situations, and physiological characteristics
- Individualized plans: Developed targeted collection strategies based on patient-specific circumstances
- Technical innovation: Used ultrasound guidance and other new technologies to improve success rates
- Safety consciousness: Highly emphasized bleeding risk with corresponding preventive measures
Experience insights:
- Elderly patient collection requires more patience and skill
- Patients on multiple medications need special attention to drug effects on test results
- Safety is always paramount – time investment is worthwhile to ensure patient safety
- Multidisciplinary collaboration is crucial for complex case management
International Standards and Development Trends
Role of International Standardization Organizations
Global Impact of CLSI Standards
The Clinical and Laboratory Standards Institute (CLSI) collection standards have become important global clinical laboratory references. CLSI GP41-A6 “Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture” is currently the most authoritative collection guidance document.
This standard details all collection aspects including patient preparation, tube selection, collection order, mixing methods, and sample handling. Standard development is based on extensive scientific research and clinical practice, possessing strong scientific validity and practicality.
WHO Global Initiatives
The World Health Organization (WHO) promotes global collection safety and standardization. WHO’s “Global Policy on Safe Injection and Related Procedures” particularly emphasizes infection control and occupational exposure protection during collection processes.
WHO also promotes developing country collection technique standardization through technical assistance and training programs, helping these countries establish internationally standard collection systems.
Comparison and Integration of National Standards
European Union Standards
The European Committee for Standardization (CEN) developed EN 14820 “Requirements for Blood Collection Systems,” essentially consistent with CLSI standards in technical requirements but differing in certain details.
For example, regarding tube color coding, EU standards allow more color variations to accommodate different manufacturer product characteristics. In quality control, EU standards emphasize traceability and documentation management more strongly.
Asian Standards Development
The Japanese Committee for Clinical Laboratory Standards (JCCLS) developed Japanese-characteristic collection standards. These standards particularly address Asian population physiological characteristics like finer vessels and thinner skin, making corresponding technical adjustments.
China’s “Clinical Blood Collection Specifications” (WS/T 661-2020) was developed by absorbing international advanced experience while combining Chinese actual conditions. The specifications maintain consistency with international standards in basic principles while reflecting Chinese characteristics in certain operational details.
Standardization Needs for Emerging Technologies
Molecular Diagnostics Collection Requirements
With rapid molecular diagnostics development, collection requirements continuously increase. Nucleic acid testing requires preventing DNA/RNA degradation, demanding special preservatives and storage conditions.
Circulating tumor DNA (ctDNA) testing requires extremely high sample quality – any DNA contamination could affect results. This necessitates specialized collection standards including special tubes, strict aseptic procedures, and specific handling processes.
Precision Medicine Personalized Needs
In the precision medicine era, genetic testing, drug sensitivity testing, and other personalized medical projects continuously increase. These tests have highly specific sample requirements, necessitating corresponding collection standards.
For example, certain genetic polymorphism testing requires avoiding DNA degradation and contamination; drug concentration monitoring requires collection at specific time points; certain biomarker testing needs special sample handling conditions.
Educational Training System Construction
Tiered Training Systems
Basic Theory Training
All personnel engaged in collection work need systematic basic theory training. Training content should include:
- Basic blood physiology knowledge
- Various tube mechanisms and applications
- Anticoagulant types and characteristics
- Blood preservation and transport principles
Theory training should use multiple formats including classroom instruction, online learning, and case analysis. Training effectiveness needs assessment through examinations – only those passing exams can proceed to next training phases.
Skills Operation Training
Skills training is the core of collection education. Training content includes:
- Standardized collection operational procedures
- Collection techniques for different patient types
- Emergency situation handling
- Specific quality control measures
Skills training should occur in simulated environments using mannequins or volunteers for practice. Training processes require experienced instructors for on-site guidance and correction.
Continuing Education and Updates
Medical knowledge and technology continuously develop, and collection standards and requirements continuously update. Therefore, continuing education systems must be established for regular personnel knowledge update training.
Continuing education content should include:
- New technology and method introductions
- International standard updates
- Quality improvement experience sharing
- Adverse event case analyses
Assessment and Evaluation Systems
Theory Assessment
Theory assessment should cover all important collection knowledge points including:
- Various tube characteristics and applications
- Collection order and mixing methods
- Common problem handling
- Quality control requirements
Assessment methods can use written tests, online examinations, or oral tests. Assessment standards should be unified, ensuring all personnel meet basic requirements.
Skills Assessment
Skills assessment directly evaluates collection abilities. Assessment content should include:
- Standardized operational procedure execution
- Collection success rates
- Sample quality (hemolysis rates, clotting rates)
- Patient satisfaction
Skills assessment should occur in actual work environments using real patients and clinical situations. Assessment standards should be quantified for easy evaluation and comparison.
Continuous Monitoring
Establish continuous monitoring systems for regular healthcare personnel collection quality assessment. Monitoring indicators should include:
- Collection success rates
- Sample rejection rates
- Patient complaint rates
- Occupational exposure incident rates
Monitoring results should be promptly fed back to relevant personnel; those with substandard quality indicators should receive targeted retraining.
Continuous Quality Improvement Initiatives
Quality Indicator System Establishment
Core Quality Indicators
Establishing scientific quality indicator systems is the foundation of quality improvement. Core quality indicators should include:
- Sample Quality Indicators
- Hemolysis rate: Normal should be <2%
- Clotting rate: Anticoagulated tubes should be <0.5%
- Insufficient blood volume rate: Should be <1%
- Sample contamination rate: Should be <0.1%
- Operational Quality Indicators
- Collection success rate: Experienced nurses should be >95%
- Recollection rate: Should be <3%
- Specimen labeling error rate: Should be <0.1%
- Tube selection error rate: Should be <0.5%
- Service Quality Indicators
- Patient satisfaction: Should be >90%
- Patient complaint rate: Should be <1%
- Wait times: Outpatients should be <30 minutes
- Result reporting time: Emergency tests should be within specified timeframes
Data Collection and Analysis
Establish comprehensive data collection systems for systematic quality indicator data collection. Data sources should include:
- Laboratory specimen quality records
- Nursing department operational quality statistics
- Patient services satisfaction surveys
- Infection control department adverse event reports
Data analysis should use statistical methods to identify root causes of quality problems. Through trend analysis, comparative analysis, and regression analysis, identify key factors affecting quality.
Continuous Improvement PDCA Cycles
Plan Phase
Based on quality indicator analysis results, develop specific improvement plans. Improvement plans should include:
- Clear improvement objectives
- Specific improvement measures
- Responsibility assignments and timelines
- Expected outcomes and evaluation criteria
Improvement plan development should follow SMART principles (Specific, Measurable, Achievable, Relevant, Time-bound) ensuring plan operability.
Do Phase
Strictly execute all measures according to improvement plans. During execution:
- Strengthen training and promotion
- Provide necessary resource support
- Establish incentive and constraint mechanisms
- Promptly resolve execution problems
Execution processes need detailed documentation to provide evidence for subsequent effectiveness evaluation.
Check Phase
Regularly examine improvement measure implementation and effectiveness. Examination content includes:
- Measure execution levels
- Quality indicator changes
- Expected objective achievement
- Unexpected problem emergence
Examination results need prompt feedback to relevant personnel, providing evidence for next actions.
Act Phase
Based on examination results, determine next actions:
- If effects are good, standardize improvement measures
- If effects are poor, analyze causes and adjust measures
- Identify new improvement opportunities, initiating next PDCA cycles
Benchmarking and Best Practices Sharing
Learning Advanced Domestic and International Experiences
Actively learn from advanced domestic and international hospital experiences and practices. Through literature research, site visits, and academic exchanges, understand latest technological developments and management concepts.
Focus areas include:
- New technology application experiences
- Quality management innovative practices
- Patient service improvement measures
- Effective cost control methods
Internal Best Practice Sharing
Establish internal best practice sharing mechanisms encouraging departments to share successful experiences. Sharing content can include:
- Technical innovation cases
- Quality improvement achievements
- Patient service highlights
- Team building experiences
Sharing formats can use case publications, experience exchange meetings, and skills competitions.

Future Outlook and Development Directions
Technology Development Trend Predictions
Artificial Intelligence Applications in Blood Collection
AI technology is gradually penetrating all medical fields, including blood collection. Future development directions may include:
- Intelligent vascular recognition systems: Using computer vision technology to automatically identify optimal collection sites, improving collection success rates.
- Collection robots: AI-enhanced collection robots will become more intelligent, automatically adjusting collection strategies based on patient-specific conditions.
- Quality prediction systems: Through machine learning algorithms, predict sample quality issues and take preventive measures in advance.
Personalized Medicine Impact
With precision medicine development, personalized medicine will create new collection requirements:
- Gene-directed collection strategies: Develop personalized collection plans based on patient genotypes.
- Pharmacogenomic testing: Require special collection and processing procedures to ensure test accuracy.
- Disease susceptibility assessment: Certain hereditary disease screening requires special collection techniques.
Standardization Process Advancement
Global Standards Unification
The future will see further internationalization and unification of collection standards:
- Global color coding unification: Collection tube color coding will achieve global uniformity.
- Quality standards unification: Pre-analytical quality control standards will tend toward international consistency.
- Training certification mutual recognition: Different countries and regions’ collection skill certifications will achieve mutual recognition.
Digital Standards Establishment
With information technology development, digital standards will become new development directions:
- Electronic operation guidelines: Collection standard operating procedures will achieve electronization and intelligence.
- Real-time quality monitoring: Through IoT technology, achieve real-time collection quality monitoring.
- Big data applications: Use big data technology to analyze collection quality influencing factors, guiding continuous standard optimization.
Educational Training Innovation
Virtual Reality Technology Applications
VR technology will bring revolutionary changes to collection training:
- Immersive learning environments: Students can practice repeatedly in virtual environments, improving learning efficiency.
- Complex case simulations: Can simulate various complex clinical situations, improving response capabilities.
- Personalized training: Provide personalized training content based on student learning progress and characteristics.
Mobile Learning Platforms
Mobile internet technology will make collection education more convenient and efficient:
- Anytime, anywhere learning: Healthcare personnel can use fragmented time for learning, improving learning flexibility.
- Interactive learning: Through gamification and socialization, improve learning interest and participation.
- Real-time updates: Latest knowledge and standards can be promptly pushed to learning platforms, ensuring knowledge timeliness.
Conclusion: Moving Toward Higher Quality Blood Collection Services
Reviewing our comprehensive analysis of clinical blood collection tube types, from basic theoretical knowledge to practical clinical applications, from traditional operational methods to future technological developments, we clearly see that blood collection – this seemingly simple medical procedure – actually contains profound scientific principles and rich technical content.
Re-emphasizing Core Points
Scientific Foundation is Key
Every blood collection tube design has strict scientific rationale. EDTA’s chelation mechanism, heparin’s anticoagulation principles, and sodium fluoride’s glycolysis inhibition are not arbitrary choices but based on in-depth biochemical and physiological research. Only by deeply understanding these scientific principles can correct choices be made in clinical practice.
Standardization is Guarantee
Tube color coding, collection order arrangement, and mixing method determination are standardized requirements validated through extensive clinical practice. Strict adherence to these standards is the basic prerequisite for ensuring test result accuracy. Any deviation from standards may lead to unpredictable consequences.
Personalization is the Development Direction
While standardization is foundational, individualized collection strategies are equally important when facing complex and diverse clinical situations. Emergency patients, pediatric patients, and elderly patients – different populations require different approaches. Future development will increasingly focus on personalized medicine needs.
Continuous Improvement is the Driving Force
Medicine is a continuously developing discipline, and collection technology is also progressing continuously. New test items, new technical methods, and new quality requirements all drive continuous improvement of collection standards. Only by maintaining attitudes of continuous learning and improvement can we keep pace with the times.
Recommendations for Healthcare Personnel
Master Solid Basic Knowledge
Every healthcare professional engaged in collection work should solidly master relevant basic knowledge. This includes not only various tube characteristics and applications but also blood physiology and basic laboratory medicine principles. Only with solid theoretical foundations can one excel in practice.
Strictly Follow Operating Standards
Standardized operating procedures are basic requirements for ensuring collection quality. No matter how skilled the operator, procedures should not be performed based on experience or intuition but should strictly follow standards at every step.
Maintain Learning Attitudes
Medical knowledge and technology continuously develop, and collection standards and requirements continuously update. Healthcare personnel should maintain lifelong learning attitudes, staying current with latest developments and continuously improving professional levels.
Emphasize Patient Experience
Collection is not only a technical procedure but also a service process. Healthcare personnel should consider issues from patients’ perspectives, minimizing patient discomfort and providing humanized service.
Recommendations for Healthcare Institutions
Establish Comprehensive Management Systems
Healthcare institutions should establish comprehensive collection management systems including standardized operational procedures, complete training systems, and effective quality control mechanisms. Management systems should cover the entire collection process from personnel training to equipment management, from quality monitoring to continuous improvement.
Invest Necessary Resources
High-quality collection services require necessary resource investment including advanced equipment, quality consumables, and adequate staffing. Healthcare institutions should consider long-term development perspectives, investing wisely in collection quality.
Foster Positive Cultural Environments
Quality culture is an important factor ensuring collection quality. Healthcare institutions should foster cultures emphasizing quality and pursuing excellence, enabling every employee to recognize collection quality importance and consciously maintain and improve service quality.
Strengthen Multidisciplinary Collaboration
Collection involves multiple departments including clinical units, nursing, and laboratories, requiring strengthened multidisciplinary collaboration. Establish effective communication mechanisms to promptly resolve work problems and collectively improve overall collection service levels.
Looking Forward
With continuous medical technology development, the collection field will also welcome new transformations. Artificial intelligence, robotics, personalized medicine, and other emerging technologies will bring new opportunities and challenges to collection.
In this transformative era, we must maintain open attitudes toward new technologies while actively embracing change, yet persist in scientifically rigorous spirits, ensuring every new technology application has adequate scientific rationale and safety guarantees.
Collection may be small, but it concerns the big picture. A small collection tube carries patient expectations, relates to diagnostic accuracy, and affects treatment effectiveness. Let us provide high-quality collection services for patients with more professional attitudes, more rigorous spirits, and more humanized service, contributing to continuous healthcare quality improvement.
As the ancients said: “Do not fail to do good even if it’s small; do not do wrong even if it’s small.” In the seemingly ordinary position of blood collection, each person’s professional performance affects overall medical service quality. Let us start with each collection, focus on every detail, and collectively build safer, more accurate, and more humanized healthcare service systems.
Though collection tube types are limited, the scientific content behind them is unlimited; though collection actions are simple, the responsibilities they carry are significant. Through our collective efforts, clinical collection quality and standards will continuously improve, making greater contributions to protecting people’s health.
Author’s Note: This article is based on current medical knowledge and clinical practice. As medical technology develops, related standards and requirements may be updated. Readers should use the latest industry standards and institutional guidelines in actual work, applying content in combination with specific clinical situations.






