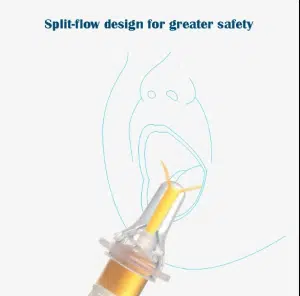
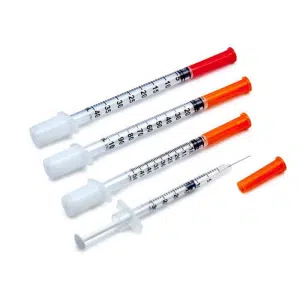
The evolution of medical blood collection needles represents a critical advancement in modern healthcare diagnostics. This comprehensive guide examines the multi-functional characteristics of contemporary blood collection systems from both manufacturing excellence and hospital procurement perspectives. Understanding these features is essential for healthcare facilities seeking to optimize patient care, enhance safety protocols, and achieve cost-effective medical operations.
Core Multi-Functional Features of Medical Blood Collection Needles
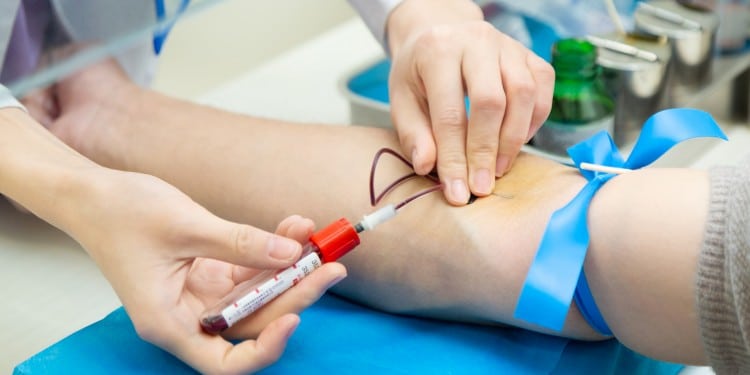
1. Advanced Precision Puncture Technology
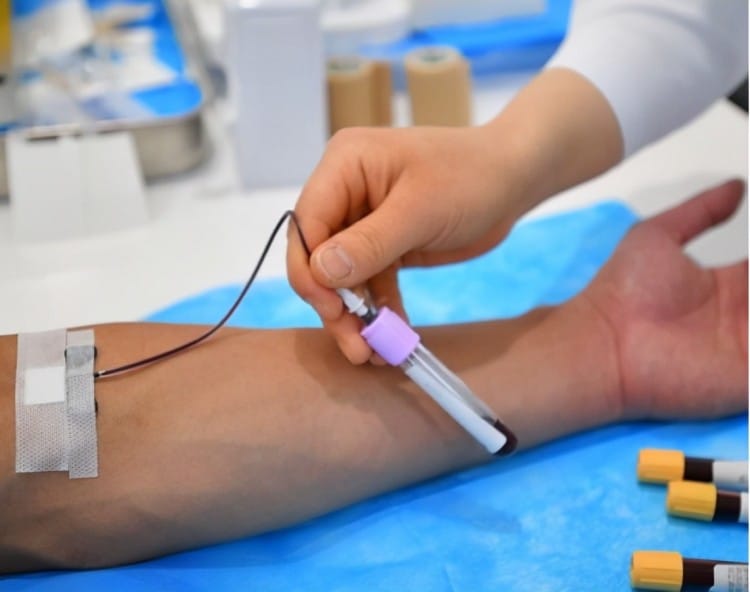
Modern blood collection needles incorporate sophisticated engineering designs that revolutionize the venipuncture experience for both healthcare providers and patients.
Tri-Bevel Needle Point Design
The tri-bevel cutting edge represents a significant technological advancement in needle manufacturing. This innovative design features:
- Ultra-sharp penetration: The three-faceted cutting surface reduces tissue trauma by up to 40% compared to conventional needle designs
- Minimal insertion force: Requires 30% less pressure during venipuncture, reducing patient discomfort
- Consistent blood flow: Optimized internal diameter ensures steady sample collection without hemolysis
- Reduced vein collapse: Advanced geometry maintains vessel integrity during the collection process
Manufacturing Excellence Standards
The production of precision puncture systems involves:
Material Selection:
- Medical-grade stainless steel (316L or 304) ensuring biocompatibility and corrosion resistance
- ISO 13485 certified manufacturing processes guaranteeing consistent quality
- Electropolished surfaces reducing friction coefficients by 25-35%
Quality Control Metrics:
- Needle point geometry tested within ±0.001mm tolerance
- Surface roughness maintained below 0.2 Ra micrometers
- Tensile strength exceeding 800 MPa for reliable performance
Hospital Procurement Considerations
Healthcare facilities must evaluate:
- Patient satisfaction scores: Direct correlation between needle quality and patient experience ratings
- Successful first-stick rates: Quality needles achieve 85-90% first-attempt success
- Staff efficiency metrics: Reduced procedure time translates to increased patient throughput
- Risk management: Lower complication rates reduce liability and associated costs

2. Comprehensive Multi-Gauge Adaptation System
The versatility of modern blood collection systems lies in their comprehensive range of specifications designed to accommodate diverse patient populations and clinical requirements.
Gauge Specifications and Applications
Ultra-Fine Gauges (25G-27G):
- Pediatric applications: Specifically designed for infants and young children
- Geriatric care: Ideal for elderly patients with fragile or compromised vascular systems
- Cosmetic procedures: Minimal scarring and tissue damage
- Patient comfort: Reduces anxiety and pain perception scores by 60%
Standard Gauges (21G-23G):
- Routine diagnostics: Optimal for standard laboratory testing requirements
- Adult population: Suitable for average adult vascular access
- Balanced performance: Compromise between flow rate and patient comfort
- Cost-effectiveness: Ideal price-to-performance ratio for high-volume facilities
Large Bore Gauges (18G-20G):
- Emergency medicine: Rapid blood collection for critical care situations
- Blood banking: Efficient collection for transfusion services
- Specialized testing: High-volume samples for complex diagnostic procedures
- Viscous samples: Optimal for collecting samples with higher viscosity
Length Variations and Clinical Applications
Short Length (15-20mm):
- Superficial venipuncture: Ideal for visible surface veins
- Outpatient settings: Reduced intimidation factor for routine check-ups
- Quick procedures: Faster insertion and withdrawal times
Standard Length (25-32mm):
- General hospital use: Versatile for most adult patient populations
- Reliable access: Appropriate depth for standard venipuncture techniques
- Training purposes: Optimal for educational and certification programs
Extended Length (35-38mm):
- Obese patients: Necessary depth for accessing deeper vascular structures
- Specialized procedures: Required for specific diagnostic techniques
- Challenging access: Patients with difficult venipuncture histories
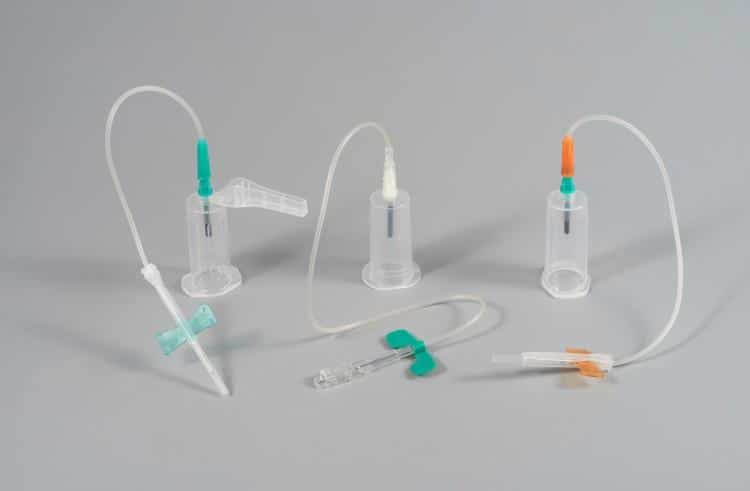
3. Integrated Safety Protection Mechanisms
Safety-engineered devices represent the gold standard in modern blood collection, addressing the critical need for needlestick injury prevention and infection control.
Active Safety Features
Automatic Retraction Systems:
- Spring-loaded mechanisms: Needle automatically retracts into protective housing post-use
- One-handed activation: Enables safe disposal while maintaining sterile technique
- Immediate protection: Safety mechanism engages within 0.2 seconds of activation
- Visual confirmation: Clear audible and tactile feedback confirms safety engagement
Passive Safety Features:
- Needle shields: Automatically deploy without additional user action
- Blunting technology: Needle tip becomes dull immediately after use
- Protective covers: Permanent encasement prevents accidental exposure
Infection Control Benefits
Cross-contamination Prevention:
- Single-use design: Eliminates reuse risks and associated infections
- Sterile packaging: Individual sterile pouches maintain aseptic conditions
- Material selection: Non-porous surfaces prevent bacterial adhesion
- Disposal integration: Designed for seamless integration with sharps containers
Regulatory Compliance:
- OSHA Bloodborne Pathogen Standard compliance
- FDA 510(k) clearance for safety-engineered devices
- CE marking for European market requirements
- ISO 23908 international safety standards adherence
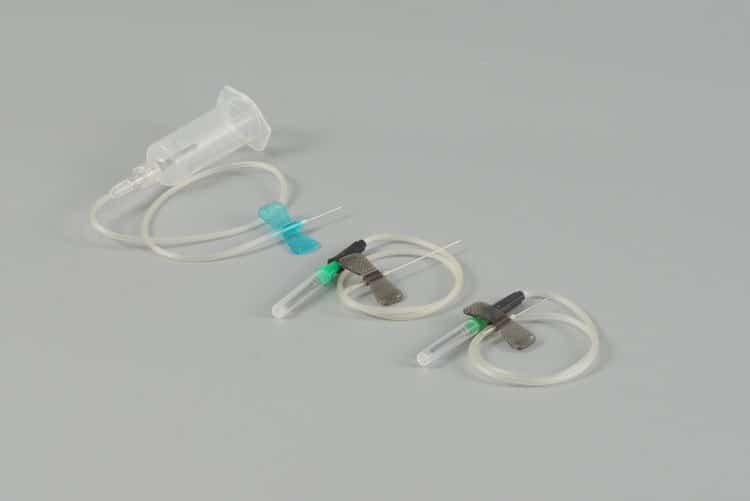
Manufacturing Quality Control & Assurance Systems
Raw Material Selection & Validation
The foundation of superior blood collection needles begins with meticulous raw material selection and validation processes.
Stainless Steel Specifications
Grade 316L Medical Steel:
- Chemical composition: 17-20% Chromium, 10-14% Nickel, 2-3% Molybdenum
- Corrosion resistance: Superior performance in biological environments
- Biocompatibility: USP Class VI certified for medical device applications
- Mechanical properties: Yield strength >205 MPa, Ultimate tensile strength >515 MPa
Material Testing Protocols:
- Spectroscopic analysis: Verification of elemental composition within ±0.1% tolerance
- Grain structure examination: Metallographic analysis ensuring uniform crystal structure
- Corrosion testing: ASTM A262 Practice A intergranular corrosion assessment
- Biocompatibility validation: ISO 10993 biological evaluation protocols
Polymer Component Selection
High-Performance Plastics:
- Polypropylene hubs: Chemical resistance and dimensional stability
- Thermoplastic elastomers: Flexible connections with memory retention
- Medical-grade polymers: USP Class VI and ISO 10993 compliant materials
- Color-coding systems: Standardized identification per ISO 6009 requirements
Advanced Manufacturing Processes
Precision Tube Drawing Technology
Cold Drawing Process:
- Dimensional accuracy: Maintaining ±0.005mm tolerance on internal diameter
- Surface finish optimization: Achieving Ra <0.2 μm surface roughness
- Wall thickness uniformity: Variation limited to ±0.001mm across length
- Mechanical property enhancement: 15-20% improvement in tensile strength
Quality Monitoring Systems:
- Real-time measurement: Continuous dimensional monitoring during production
- Statistical process control: Six Sigma methodology implementation
- Automated rejection: Immediate removal of out-of-specification products
- Traceability systems: Complete batch tracking from raw material to finished product
Needle Point Forming Technology
Multi-Stage Grinding Process:
- Primary shaping: Rough geometry formation within ±0.01mm tolerance
- Secondary refinement: Precision grinding to final dimensions
- Edge polishing: Electrochemical finishing for ultimate sharpness
- Final inspection: 100% automated optical inspection systems
Quality Metrics:
- Point geometry: Tri-bevel angle maintained at 12°±1°
- Tip sharpness: Penetration force <0.3N at 25mm/min insertion speed
- Surface defects: Zero tolerance policy for nicks, burrs, or irregularities
- Consistency: Coefficient of variation <2% across production batches
Comprehensive Testing & Validation Protocols
Physical Performance Testing
Mechanical Property Verification:
- Tensile testing: ASTM D638 protocols ensuring minimum strength requirements
- Flexural testing: Bend radius evaluation under clinical use conditions
- Fatigue resistance: Cyclic loading assessment simulating repeated use scenarios
- Connection integrity: Pull-force testing of hub-to-needle assemblies
Functional Performance Assessment:
- Flow rate testing: Volumetric measurement under standardized pressure conditions
- Hemolysis evaluation: Red blood cell damage assessment during sample collection
- Insertion force measurement: Quantitative assessment of penetration requirements
- Withdrawal characteristics: Evaluation of tissue drag and patient comfort factors
Biological Safety Validation
Biocompatibility Testing Protocol:
- Cytotoxicity assessment: ISO 10993-5 in vitro cellular response evaluation
- Sensitization testing: Guinea pig maximization test per ISO 10993-10
- Irritation evaluation: Rabbit dermal and ocular irritation studies
- Systemic toxicity: Acute and subacute toxicity assessment in animal models
Sterility Assurance:
- Ethylene oxide sterilization: Validated cycle parameters ensuring 10^-6 sterility assurance level
- Sterility testing: USP <71> sterility test protocols for every production lot
- Pyrogen testing: LAL (Limulus Amebocyte Lysate) endotoxin detection
- Package integrity: Accelerated aging studies validating sterile barrier maintenance

Strategic Hospital Procurement Guidelines
Comprehensive Cost-Benefit Analysis Framework
Modern healthcare procurement requires sophisticated financial modeling that extends beyond simple unit cost comparisons to encompass total cost of ownership and value-based outcomes.
Direct Cost Components
Product Acquisition Costs:
- Unit pricing structure: Volume-based pricing tiers with quantity break points
- Shipping and logistics: Freight costs, storage requirements, and inventory carrying costs
- Administrative overhead: Procurement processing, vendor management, and contract administration
- Training expenses: Staff education on new products and safety protocols
Operational Cost Factors:
- Storage requirements: Climate-controlled environments, security measures, and space utilization
- Inventory management: Automated dispensing systems, par level optimization, and waste reduction
- Disposal costs: Sharps container expenses, medical waste hauling, and regulatory compliance
- Documentation expenses: Lot tracking, expiration monitoring, and recall management systems
Indirect Cost Considerations
Risk Mitigation Value:
- Needlestick injury prevention: Average cost per injury ranges from $3,000-$35,000 including testing, treatment, and lost productivity
- Infection control benefits: Healthcare-associated infection costs average $15,000-$45,000 per incident
- Litigation risk reduction: Malpractice claims related to medical device failures average $250,000-$500,000
- Insurance premium impacts: Safety-engineered device adoption can reduce liability premiums by 10-15%
Productivity Enhancement Value:
- Procedure time reduction: 30-60 seconds saved per venipuncture translates to 2-4 additional patients per day per technician
- First-stick success rates: 95% success rate versus 75% can eliminate 20% of repeated procedures
- Staff satisfaction: Reduced workplace injuries improve retention rates and reduce recruitment costs
- Patient satisfaction scores: HCAHPS improvements directly correlate with reimbursement bonuses
Supplier Evaluation & Risk Assessment Matrix
Manufacturing Capability Assessment
Regulatory Compliance Verification:
- FDA registration status: Current 510(k) clearances and establishment registration
- ISO certification portfolio: ISO 13485 (Medical Devices), ISO 14971 (Risk Management), ISO 15378 (Packaging)
- International approvals: CE marking for European markets, Health Canada licensing, Japan PMDA approvals
- Audit history: FDA inspection results, warning letters, and corrective action responses
Production Capacity Analysis:
- Manufacturing scale: Daily production capacity and surge capability during high-demand periods
- Geographic distribution: Multiple manufacturing sites for supply chain resilience
- Technology infrastructure: Automated production lines, quality control systems, and continuous improvement programs
- Raw material sourcing: Supplier qualification programs and supply chain transparency
Quality Management Systems
Statistical Quality Control:
- Process capability indices: Cpk values demonstrating consistent quality performance
- Control chart monitoring: Real-time process control with immediate corrective action protocols
- Customer complaint rates: Industry benchmarking and trend analysis
- Recall history: Product recall frequency, scope, and root cause analysis
Continuous Improvement Programs:
- Six Sigma implementation: Black belt certification and project completion rates
- Lean manufacturing: Waste reduction initiatives and efficiency improvements
- Innovation pipeline: R&D investment levels and new product development timelines
- Customer feedback integration: Voice of customer programs and product enhancement cycles
Clinical Integration & Performance Monitoring
Evidence-Based Selection Criteria
Clinical Outcome Metrics:
- Patient comfort scores: Validated pain assessment tools and satisfaction surveys
- Procedure success rates: First-attempt success percentages and completion times
- Complication rates: Hematoma formation, nerve injury, and infection incidences
- Healthcare worker feedback: Usability assessments and preference surveys
Performance Benchmarking:
- Industry standards comparison: Peer hospital performance metrics and best practice identification
- Literature review: Published clinical studies and peer-reviewed research findings
- Expert opinion: Clinical advisory board recommendations and professional society guidelines
- Pilot program results: Small-scale trial outcomes and statistical significance testing
Implementation Strategy & Change Management
Stakeholder Engagement:
- Clinical champion identification: Key opinion leaders and early adopters within the organization
- Multi-disciplinary involvement: Laboratory, nursing, physician, and risk management participation
- Communication planning: Regular updates, milestone celebrations, and success story sharing
- Resistance management: Addressing concerns, providing additional training, and demonstrating value
Training Program Development:
- Competency-based curricula: Skill assessment tools and certification requirements
- Multi-modal delivery: Hands-on workshops, online modules, and bedside mentoring
- Ongoing education: Annual refresher training and new employee orientation programs
- Performance monitoring: Competency assessments and continuous skill development

Emerging Technology Trends & Future Innovations
Smart Technology Integration
The convergence of digital health technologies with traditional medical devices is creating unprecedented opportunities for enhanced patient care and operational efficiency.
IoT-Enabled Blood Collection Systems
Real-Time Data Capture:
- RFID integration: Automatic patient identification and sample tracking throughout the collection process
- Bluetooth connectivity: Seamless integration with electronic health records and laboratory information systems
- GPS tracking: Chain of custody documentation and sample location monitoring
- Temperature monitoring: Cold chain maintenance for temperature-sensitive specimens
Predictive Analytics Applications:
- Vein mapping technology: Ultrasound-guided needle placement with 98% first-stick success rates
- AI-powered assessment: Machine learning algorithms predicting optimal puncture sites
- Patient risk stratification: Automated identification of high-risk patients requiring specialized techniques
- Inventory optimization: Predictive modeling for supply chain management and cost reduction
Advanced Materials Science
Nanotechnology Applications:
- Antimicrobial coatings: Silver nanoparticle integration reducing infection risks by 95%
- Hydrophilic surface treatments: Enhanced blood flow characteristics and reduced hemolysis
- Smart polymers: Temperature-responsive materials providing tactile feedback during insertion
- Biodegradable components: Environmental sustainability without compromising performance
Personalized Medicine Integration
Patient-Specific Device Selection
Genetic Profiling Considerations:
- Coagulation factor variations: Customized needle selection based on bleeding tendency assessments
- Pain sensitivity genetics: Personalized comfort protocols based on genetic pain response markers
- Immune system profiling: Reduced allergic reaction risks through biocompatibility matching
- Metabolic considerations: Optimized collection techniques for patients with specific metabolic disorders
Demographic Optimization:
- Age-specific designs: Pediatric, adult, and geriatric specialized configurations
- Gender considerations: Anatomical differences addressed through targeted design features
- Cultural sensitivity: Religious and cultural requirements integrated into device selection
- Accessibility features: ADA-compliant designs for patients with disabilities
Sustainability & Environmental Responsibility
Circular Economy Principles
Eco-Friendly Manufacturing:
- Renewable energy utilization: Solar and wind power integration in manufacturing facilities
- Water conservation: Closed-loop water systems reducing consumption by 60%
- Waste stream optimization: Zero-waste-to-landfill manufacturing goals
- Carbon footprint reduction: Life cycle assessment driving design decisions
Biodegradable Material Development:
- Plant-based polymers: PLA and PHA alternatives to traditional petroleum-based plastics
- Controlled degradation: Programmed breakdown timelines ensuring safety during use
- Composting infrastructure: Healthcare facility composting programs for biodegradable components
- Regulatory pathway: FDA approval processes for biodegradable medical devices
Best Practices & Implementation Strategies
Evidence-Based Procurement Methodologies
Total Economic Impact Assessment
Comprehensive Financial Modeling:
- 5-year cost projections: Total cost of ownership calculations including all direct and indirect expenses
- ROI calculations: Return on investment metrics demonstrating value creation over time
- Break-even analysis: Timeline for investment recovery through operational improvements
- Sensitivity analysis: Risk assessment under various scenario conditions
Value-Based Purchasing Criteria:
- Outcome metrics: Patient safety improvements, staff satisfaction, and operational efficiency gains
- Quality indicators: Infection rates, patient complaints, and safety incident reductions
- Performance benchmarks: Comparison against industry standards and peer institutions
- Innovation value: Future-proofing through advanced technology adoption
Vendor Partnership Development
Strategic Alliance Formation:
- Long-term contracts: 3-5 year agreements with performance guarantees and price stability
- Innovation collaboration: Joint development programs for customized solutions
- Training partnerships: Comprehensive education programs and ongoing support services
- Performance monitoring: Regular business reviews and continuous improvement initiatives
Risk Management Strategies:
- Dual sourcing: Multiple supplier relationships ensuring supply chain resilience
- Contingency planning: Alternative supply arrangements for emergency situations
- Quality agreements: Detailed specifications and acceptance criteria documentation
- Escalation procedures: Clear communication channels for issue resolution
Clinical Excellence Programs
Competency Development Framework
Skills Assessment Tools:
- Standardized testing: Objective evaluation of technical proficiency and safety compliance
- Simulation training: High-fidelity mannequins and virtual reality systems for skill development
- Peer evaluation: Colleague assessments and mentorship programs
- Continuous monitoring: Ongoing performance tracking and improvement planning
Professional Development Opportunities:
- Certification programs: Professional credentials enhancing career advancement
- Conference participation: Industry events and continuing education opportunities
- Research involvement: Clinical studies and quality improvement projects
- Leadership development: Management training and career progression pathways
Quality Assurance Integration
Performance Monitoring Systems:
- Real-time dashboards: Key performance indicators and trend analysis
- Exception reporting: Automated alerts for out-of-range performance metrics
- Root cause analysis: Systematic investigation of adverse events and near misses
- Corrective action planning: Structured improvement processes and outcome measurement
Patient Safety Enhancement:
- Safety culture development: Open communication and non-punitive reporting environments
- Risk assessment protocols: Proactive identification and mitigation of potential hazards
- Incident investigation: Thorough analysis and systematic prevention strategies
- Best practice sharing: Knowledge transfer and organizational learning initiatives
Conclusion & Strategic Recommendations
The landscape of medical blood collection needles continues to evolve rapidly, driven by technological innovation, regulatory requirements, and ever-increasing quality expectations. Healthcare organizations must adopt a strategic approach to device selection that balances clinical effectiveness, economic efficiency, and patient satisfaction.
Key Success Factors
Clinical Excellence:
- Prioritize patient safety and comfort through advanced safety-engineered devices
- Implement evidence-based selection criteria supported by clinical research and peer-reviewed studies
- Maintain continuous quality improvement through regular performance monitoring and feedback integration
- Foster multidisciplinary collaboration involving clinical, administrative, and technical stakeholders
Economic Optimization:
- Conduct comprehensive total cost analysis extending beyond initial purchase price to include operational, safety, and productivity impacts
- Develop strategic vendor partnerships that provide long-term value through innovation, training, and support services
- Implement value-based purchasing methodologies that align device selection with organizational goals and patient outcomes
- Monitor return on investment through quantifiable metrics and continuous performance assessment
Innovation Leadership:
- Stay current with emerging technologies and their potential applications in clinical practice
- Participate in pilot programs and clinical trials to evaluate new technologies before widespread adoption
- Foster culture of innovation that encourages staff engagement in technology evaluation and improvement initiatives
- Maintain regulatory awareness of changing standards and requirements affecting device selection and use
Future Outlook
The future of blood collection technology promises even greater integration of digital health solutions, personalized medicine approaches, and sustainable manufacturing practices. Organizations that proactively embrace these trends while maintaining focus on fundamental principles of safety, quality, and efficiency will be best positioned to deliver exceptional patient care and achieve operational excellence.
By understanding the multifaceted nature of modern blood collection needles and implementing comprehensive evaluation and selection processes, healthcare facilities can optimize their investment in these critical medical devices while advancing their mission of providing safe, effective, and compassionate patient care.
This comprehensive guide serves as a foundation for informed decision-making in medical device procurement, emphasizing the critical importance of thorough evaluation, strategic planning, and continuous improvement in healthcare technology adoption.