The global healthcare industry has been hit with game-changing disruptions over the past few years, putting massive strain on medical device supply chains worldwide. At the heart of healthcare delivery are syringes, and the syringe manufacturer producing them are wrestling with a tangled web of supply chain headaches that directly affect patient outcomes across the globe. With skyrocketing demand for medical devices and healthcare supply chain management getting more complex by the day, tackling these syringe manufacturer supply chain challenges isn’t just important—it’s mission-critical for the entire medical device manufacturing industry.
Why Syringe Manufacturers Are Essential to Global Healthcare
Syringe manufacturer companies are the unsung heroes keeping healthcare running smoothly, cranking out billions of units every year to support everything from your annual flu shot to life-saving emergency procedures. These pharmaceutical equipment suppliers have to keep production humming while juggling a maze of suppliers, red tape, and distribution networks. The COVID-19 pandemic was a real wake-up call, showing just how quickly these supply chain networks can fall apart, leaving vaccination programs and everyday medical care hanging in the balance.
Today’s syringe manufacturer operates in a heavily regulated world where cutting corners on quality, safety, or reliability simply isn’t an option. Every single piece—from the plastic tube to the rubber stopper—has to meet rock-solid medical manufacturing compliance standards while being mass-produced and delivered on time to hospitals and clinics around the world.
The Biggest Supply Chain Headaches for Syringe Manufacturers
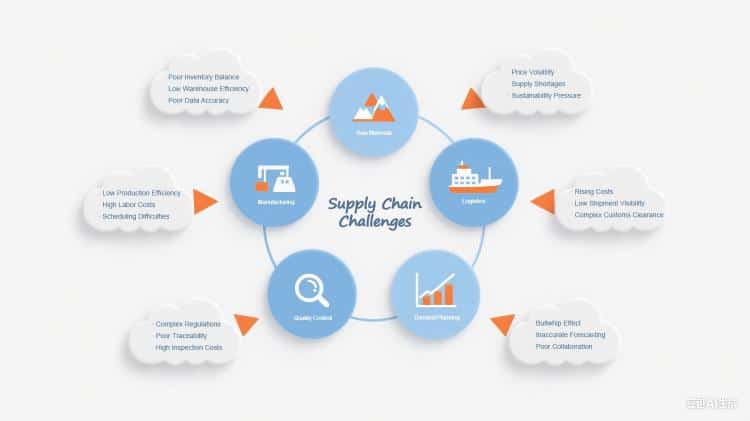
Getting Your Hands on Quality Raw Materials

The biggest pain point for any syringe manufacturer? Locking down reliable sources of top-notch raw materials. Making medical-grade syringes means you need specialized plastics, metals, and rubber that pass strict safety tests. The catch? These materials only come from a handful of pharmaceutical equipment suppliers worldwide, creating major chokepoints in the supply chain.
Since most syringe parts are petroleum-based, manufacturers get hit hard when oil prices swing or supplies get tight. Making things worse, you can’t just swap suppliers on a whim—each new vendor has to go through months of testing and regulatory approval before they can ship their first order.
Navigating Regulatory Red Tape Across Different Countries
Syringe manufacturer companies have to thread the needle through a crazy quilt of regulations that change from country to country. The FDA here in the States, Europe’s regulatory agencies, and similar watchdogs elsewhere all have their own playbooks for medical device manufacturing, quality control, and supply chain documentation.
These medical manufacturing compliance rules don’t stop at the factory door—they reach deep into the entire supply chain. Every supplier needs to be vetted, audited, and constantly monitored to stay compliant. Want to change suppliers or tweak your manufacturing process? Get ready for a mountain of paperwork and regulatory approval that can drag on for months, making it tough to pivot when problems arise.
Keeping Quality Tight and Tracking Everything

Medical device manufacturing demands bulletproof quality control from start to finish. Every syringe manufacturer needs rock-solid quality systems that can trace materials from the source all the way to the hospital loading dock. That means keeping detailed records on supplier certifications, incoming material checks, production specs, and shipping logs.
The challenge gets even trickier when you’re dealing with global healthcare logistics. Parts might start in three different countries, pass through various middlemen, and get processed at multiple facilities before ending up in your assembly line. Keeping tabs on quality and traceability across this sprawling supply chain network takes some seriously sophisticated systems.
Predicting Demand and Planning Capacity

Healthcare’s boom-and-bust cycles make demand forecasting a real nightmare for syringe manufacturer companies. Flu season, vaccination campaigns, and unexpected health crises can send demand through the roof with zero warning. COVID-19 was the perfect example—syringe demand exploded overnight as vaccination programs kicked into high gear worldwide.
Standard forecasting models often crash and burn when these demand spikes hit, leaving manufacturers either sitting on expensive excess inventory or scrambling to meet critical healthcare needs. Companies have to walk a tightrope between the cost of keeping extra capacity on standby and the risk of letting down healthcare providers when emergencies strike.
Wrestling with Global Shipping and Distribution
Getting medical devices to market involves complex global healthcare logistics that have to maintain proper temperatures, keep everything sterile, and follow shipping rules across dozens of countries. Syringe manufacturer companies serving global markets need distribution strategies that can adapt to local requirements and roll with unexpected disruptions.
Recent global chaos has shown just how fragile international shipping really is. Backed-up ports, shipping delays, and border restrictions can throw delivery schedules into complete chaos. For critical medical devices like syringes, these delays can mean the difference between life and death for patients and derail entire public health programs.
Smart Strategies for Building Bulletproof Supply Chains
Spreading Your Supplier Bets
Top syringe manufacturer companies are rolling out comprehensive supplier diversification game plans to avoid putting all their eggs in one basket. This means finding and qualifying multiple pharmaceutical equipment suppliers for critical parts across different parts of the world. Sure, it costs serious money to develop and certify all these suppliers, but it’s insurance against supply chain disasters.
The trick is balancing cost control with risk management. Manufacturers are increasingly using dual-sourcing for must-have components while keeping broader supplier networks for less critical stuff. This approach helps keep costs reasonable while ensuring healthcare supply chain management security.
Going Digital with Supply Chain Tech

Technology is becoming the secret weapon in healthcare supply chain management for syringe manufacturer companies. Advanced planning software, real-time tracking, and predictive analytics help companies spot and respond to supply chain hiccups before they become full-blown disasters.
Internet of Things sensors give you real-time eyes on inventory levels, shipping conditions, and production status throughout your entire supply chain. AI and machine learning crunch massive amounts of data to flag potential disruptions before they hit and suggest the best ways to respond.
Blockchain is emerging as a powerful tool for keeping track of everything and fighting fake products. This technology creates permanent records of where materials come from, how they’re processed, and who handles them along the way, helping manufacturers meet medical manufacturing compliance requirements while keeping counterfeit products out of the market.
Bringing Production In-House and Moving Closer to Home

Some syringe manufacturer companies are going the vertical integration route, bringing critical production steps in-house to reduce dependence on outside suppliers. This gives you more control over quality, capacity, and delivery schedules, but it requires major upfront investment and building new expertise in medical device manufacturing.
Near-shoring and reshoring are also picking up steam as manufacturers look to simplify their supply chain networks and reduce shipping risks. By setting up production closer to major markets, companies can respond faster while cutting transportation costs and environmental impact through better global healthcare logistics.
Teaming Up with Industry Partners
Modern supply chain networks are too complex for any one company to handle alone. Syringe manufacturer companies are increasingly joining industry groups and public-private partnerships designed to strengthen healthcare supply chain management resilience across the entire medical device manufacturing sector.
These collaborative efforts include shared supplier databases, joint investments in supply chain infrastructure, and coordinated emergency response plans. By working together, manufacturers can achieve better economies of scale in pharmaceutical equipment suppliers development while creating stronger supply networks.
Building Operations That Last and Stay Strong
Going Green While Staying Safe

Today’s syringe manufacturer companies face growing pressure to reduce their environmental footprint without compromising product quality or safety. This includes streamlining packaging to cut waste, implementing recycling programs, and developing more sustainable materials and processes within medical device manufacturing.
Supply chain sustainability now factors into supplier selection, with manufacturers increasingly evaluating pharmaceutical equipment suppliers based on their environmental performance and green practices. This comprehensive approach helps create more resilient supply chains while addressing environmental concerns.
Investing in People and Skills
The complexity of modern medical device manufacturing requires highly skilled workers throughout the supply chain. Syringe manufacturer companies are pouring money into workforce development programs to ensure their teams can handle complex healthcare supply chain management effectively.
This includes training on advanced planning systems, quality management principles, and medical manufacturing compliance. Many manufacturers are also building cross-functional teams that can quickly adapt to changing circumstances and support supply chain resilience initiatives.
Managing Financial Risks
Supply chain disruptions can seriously damage a syringe manufacturer’s bottom line. Companies are implementing comprehensive risk management strategies that include insurance coverage, financial hedging, and emergency funding arrangements.
These financial tools help manufacturers weather supply chain storms without compromising their ability to serve healthcare customers or invest in long-term improvements. Smart financial planning also enables companies to maintain surge capacity for emergency situations.
What’s Coming Next: Future Trends and Opportunities

Automation and Advanced Technology
The future of syringe manufacturer supply chain operations will be increasingly driven by cutting-edge technology and automation. Robotic systems, automated vehicles, and smart warehouses are transforming how materials move through production and distribution networks in medical device manufacturing.
These technologies don’t just boost efficiency and cut costs—they also improve flexibility and responsiveness. Automated systems can quickly adapt to changing production needs and help keep operations running during workforce disruptions.
Evolving Regulations and Global Standards
Regulatory agencies worldwide are working toward more unified medical device manufacturing requirements, which could simplify supply chain compliance for syringe manufacturer companies. International standards and mutual recognition agreements are gradually reducing the complexity of serving multiple markets.
However, manufacturers need to stay sharp about evolving medical manufacturing compliance requirements, particularly around supply chain security, traceability, and cybersecurity. Future regulations may impose additional requirements for healthcare supply chain management visibility and risk management.
Embracing Circular Economy Principles
The healthcare industry is starting to embrace circular economy principles, with major implications for syringe manufacturer companies and their supply chain operations. This includes designing products for recycling, implementing take-back programs, and developing closed-loop material flows.
These initiatives require close collaboration with pharmaceutical equipment suppliers, customers, and waste management partners to create sustainable material cycles. While challenging to implement, circular economy approaches can provide competitive advantages and help address environmental concerns in medical device manufacturing.
The Bottom Line
The challenges facing syringe manufacturer companies in managing their supply chain operations are complex and constantly evolving, requiring smart strategies and continuous adaptation. Success in this environment depends on building resilient, flexible, and sustainable healthcare supply chain management networks that can handle both predictable patterns and unexpected curveballs.
As the healthcare industry continues to evolve and global healthcare logistics become increasingly complex, syringe manufacturer companies must embrace innovation, collaboration, and strategic planning to ensure they can keep serving critical healthcare needs. The hard-won lessons from recent global challenges provide valuable insights for building stronger supply chains that can support global health security while maintaining operational efficiency and financial sustainability.
The road ahead means balancing multiple competing priorities: cost efficiency versus resilience, speed versus quality, innovation versus medical manufacturing compliance. Those manufacturers who successfully navigate these challenges while building sustainable, adaptable supply chain networks will be best positioned to serve the critical healthcare needs of communities worldwide.






