Introduction: What Makes Double Chamber Infusion Sets Essential in Modern Healthcare?
In today’s rapidly evolving medical landscape, the choice of an infusion set can significantly impact patient safety, treatment efficacy, and operational efficiency. The double chamber infusion set represents a breakthrough in intravenous therapy delivery, offering enhanced precision and safety features that traditional single-chamber systems cannot match. This comprehensive guide explores why healthcare professionals worldwide are transitioning to advanced disposable infusion set solutions and what factors should influence your procurement decisions.
What is a Double Chamber Infusion Set? Understanding the Technology
Core Design and Functionality
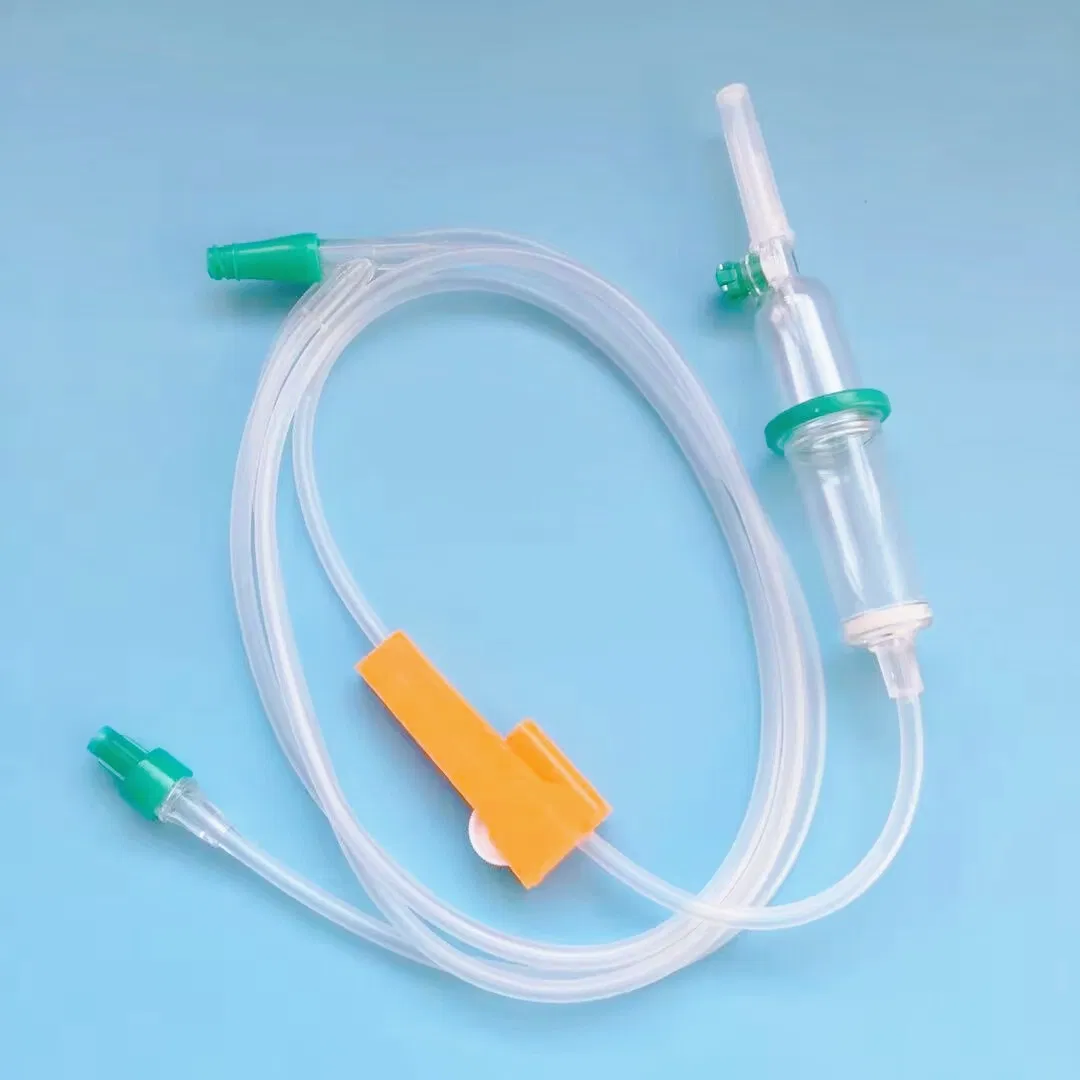
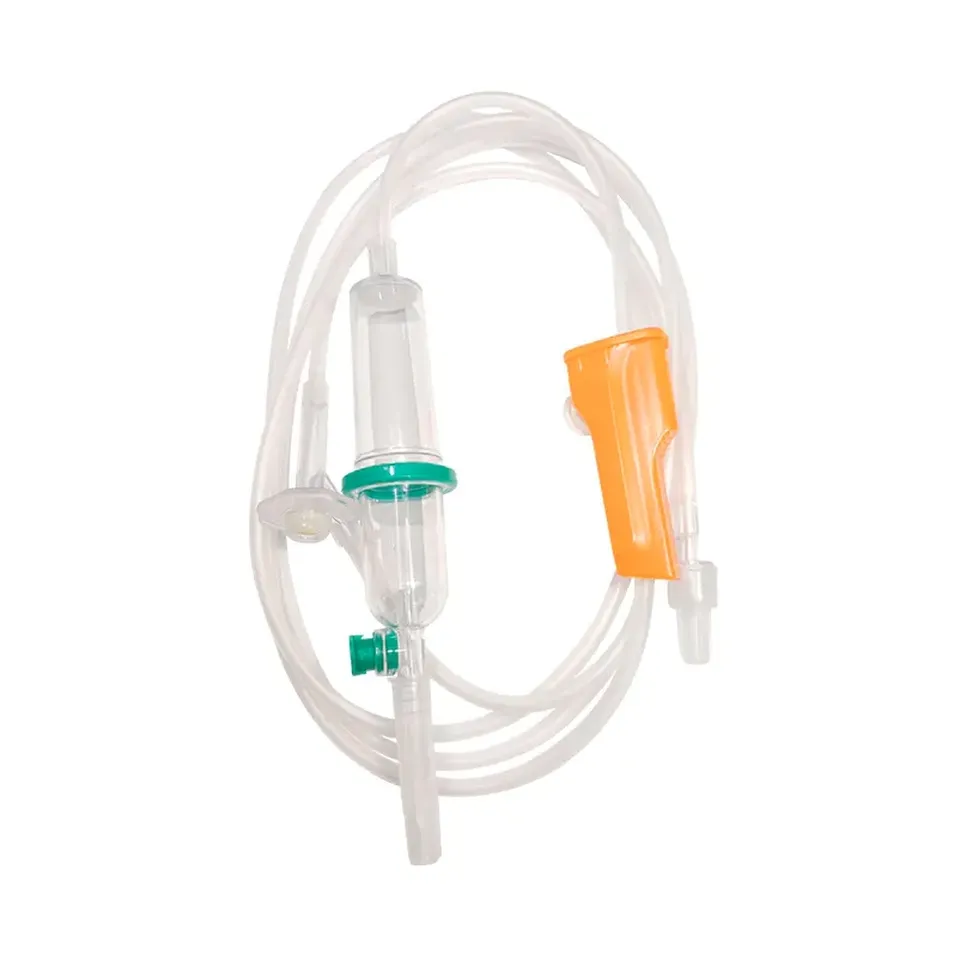
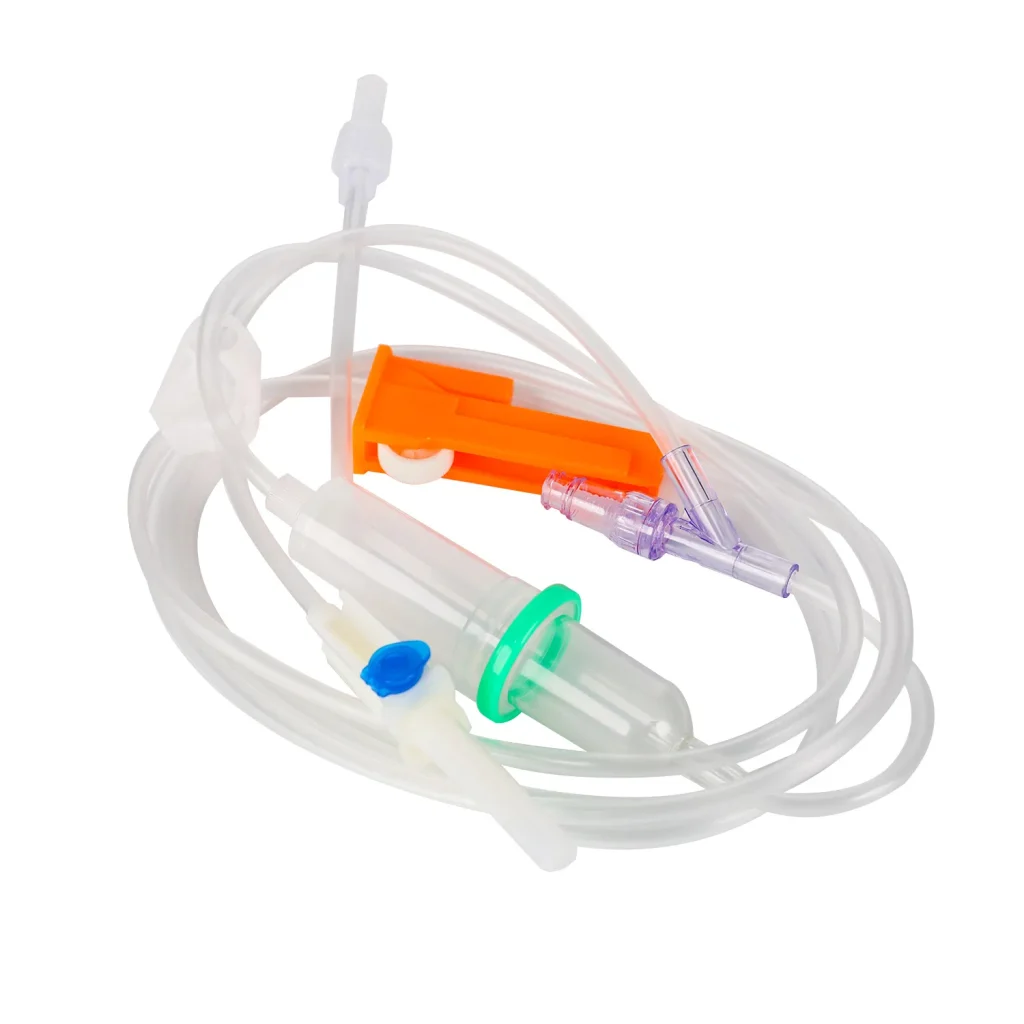
A double chamber infusion set is an advanced IV set type that incorporates two separate chambers within the drip mechanism, providing superior flow visualization and air bubble detection compared to conventional single-chamber designs. This disposable infusion set works similarly to standard intravenous lines but with enhanced safety features.
The system utilizes a sterile needle housed within a flexible cannula for subcutaneous insertion. Once the needle punctures the skin and establishes access to subcutaneous tissue, it is safely withdrawn while the cannula remains in place, minimizing patient discomfort and infection risks.
Key Components Breakdown
Every quality infusion set comprises multiple precision-engineered components:
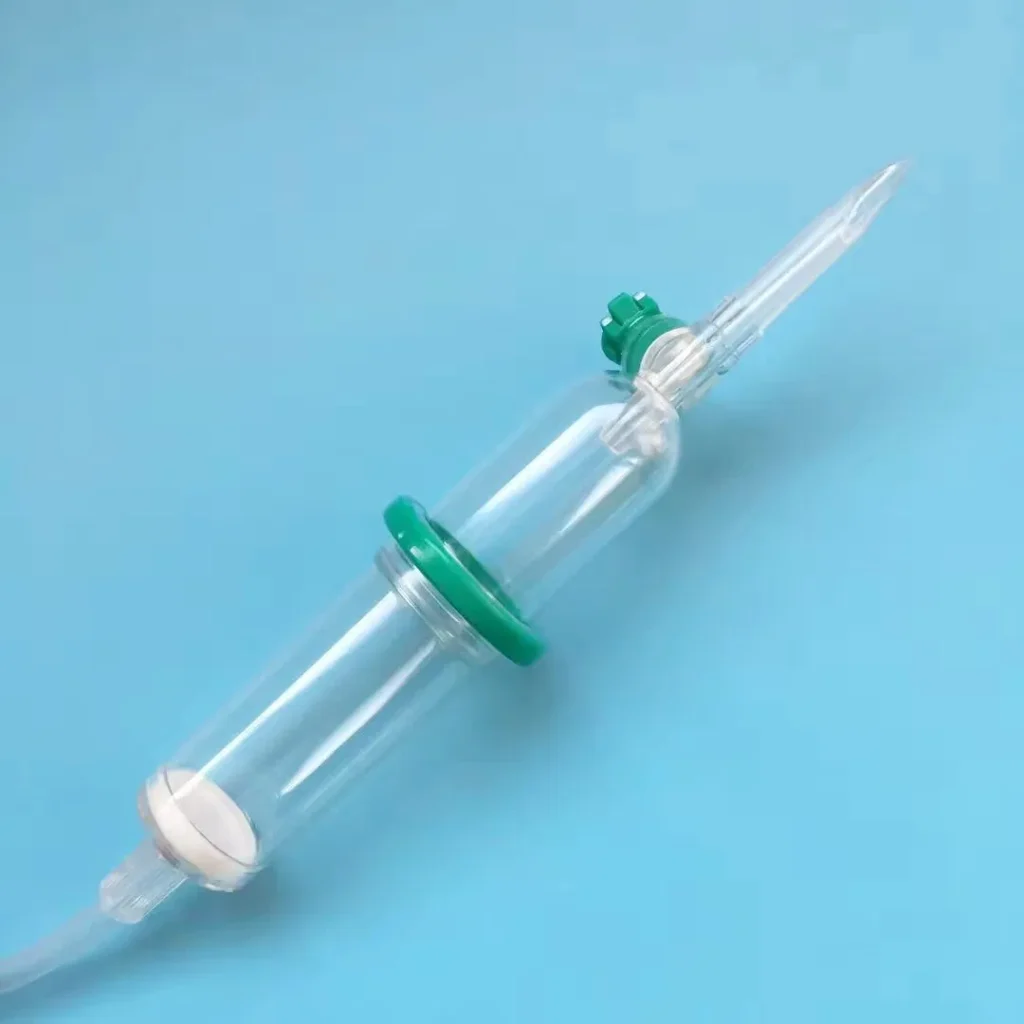
- Double Drip Chamber: Medical-grade PVC construction with transparent walls for clear flow monitoring
- Vented Spike: ABS material ensuring secure connection to IV containers
- Flow Regulator: Precision roller clamp allowing accurate adjustment (15 or 20 drops/mL options)
- Soft Tubing: Medical-grade flexible tubing available in 1.2m, 1.5m, or 1.8m lengths
- Safety Filter: Integrated micro-filtration system preventing particulate contamination
- Y-Type Injection Port: Secondary access point for medication administration
- Luer Connectors: Available in slip or lock configurations for secure attachment
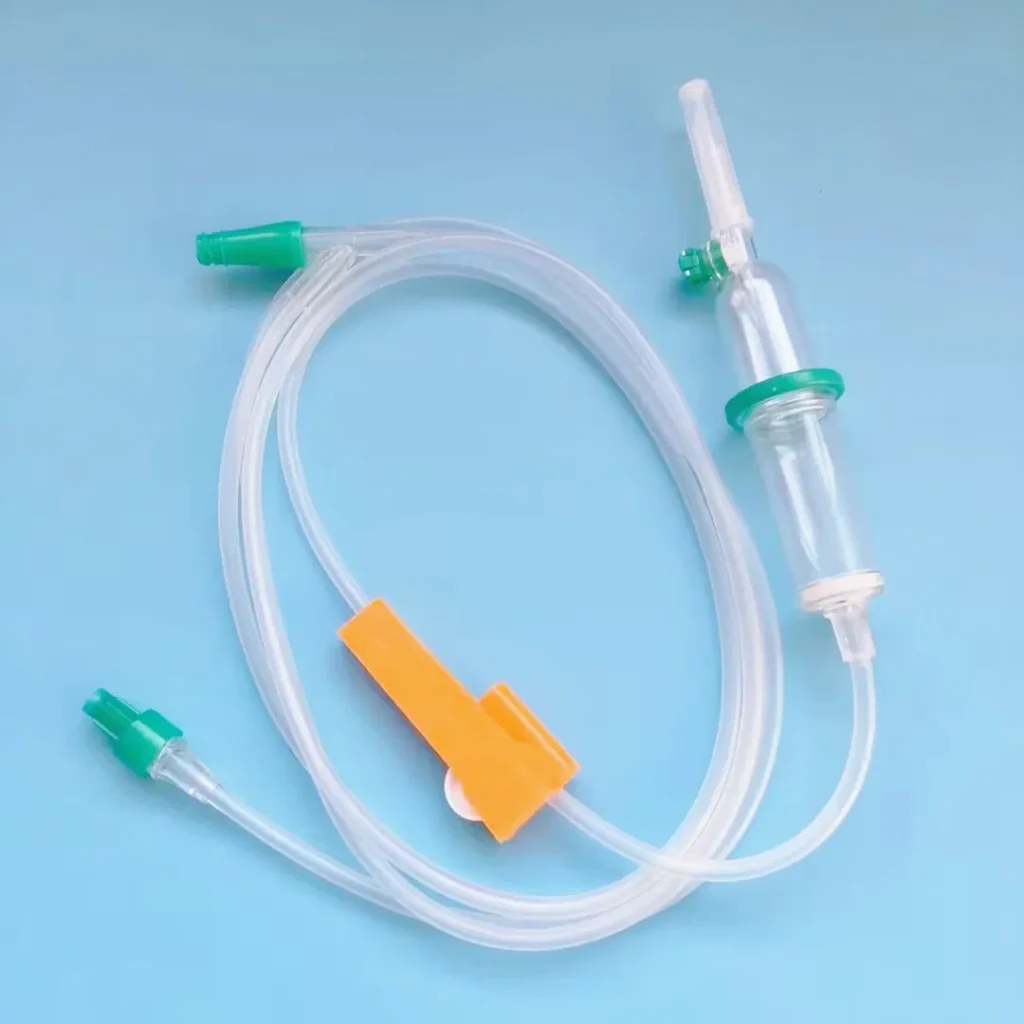
Why Choose Double Chamber Technology? The Clinical Advantages
Enhanced Patient Safety
The primary advantage of this IV set type lies in its dual-chamber design, which provides:
Superior Air Detection: The double chamber configuration makes air bubbles immediately visible, allowing healthcare providers to identify and eliminate them before they enter the patient’s bloodstream. Traditional single-chamber disposable infusion sets often obscure small air bubbles within the drip mechanism.
Auto Liquid Stop Mechanism: Advanced models incorporate automatic flow cessation when the IV container empties, preventing air embolism—a critical safety feature for busy clinical environments where continuous monitoring may be challenging.
Flow Visualization: The transparent double chamber design enables precise monitoring of drip rates, essential for medications requiring exact dosing protocols.
Clinical Versatility
Modern infusion set technology accommodates diverse clinical scenarios:
Gravity Infusion Sets (G-Type): Optimized for standard gravity-fed applications in general wards, outpatient clinics, and home healthcare settings.
Pressure Infusion Sets (P-Type): Engineered to withstand high-pressure delivery through compatible infusion pumps, ideal for emergency departments, intensive care units, and surgical theaters where rapid fluid resuscitation is necessary.
Material Safety and Biocompatibility
Quality disposable infusion set products now offer multiple material options:
- Standard Medical-Grade PVC: Cost-effective solution meeting all regulatory standards
- DEHP-FREE Formulations: Eliminates di(2-ethylhexyl) phthalate exposure concerns, particularly important for pediatric, neonatal, and pregnant patients
- TPE (Thermoplastic Elastomer): Advanced biocompatible alternative with superior flexibility and reduced environmental impact
Who Benefits from Advanced IV Set Types? Target Applications
Hospital and Clinical Settings
Acute Care Hospitals: The double chamber infusion set excels in high-acuity environments where medication errors can have severe consequences. Emergency departments, intensive care units, and surgical recovery areas benefit from enhanced flow control and safety features.
Outpatient Infusion Centers: Oncology clinics, dialysis centers, and infusion therapy facilities require reliable IV set types that minimize staff monitoring burden while maintaining patient safety during extended treatment sessions.
Pediatric Departments: Children require precise fluid management with minimal margin for error. The enhanced visualization offered by double chamber disposable infusion sets helps pediatric nurses accurately titrate fluid delivery.
Long-Term Care and Home Healthcare
Home infusion therapy continues expanding as healthcare systems shift toward outpatient treatment models. Patients receiving home parenteral nutrition, antibiotic therapy, or pain management benefit from infusion set designs that caregivers can monitor easily without specialized training.
Emergency Medical Services
Ambulance services and emergency response teams need durable, reliable IV set types that function flawlessly under challenging conditions. Pressure-rated disposable infusion sets enable rapid fluid resuscitation during patient transport.
Where Should You Source Your Infusion Sets? Manufacturing and Quality Considerations
ISO 13485 Certification: The Quality Benchmark
When selecting an infusion set supplier, ISO 13485 certification represents the global standard for medical device quality management systems. This certification ensures:
- Validated manufacturing processes with documented quality controls
- Traceability throughout the production chain
- Regular audits by independent certification bodies
- Compliance with regulatory requirements across multiple markets
CE Marking and International Compliance
For distributors serving European markets, CE marking on disposable infusion set products confirms conformity with EU Medical Device Regulations. Reputable manufacturers maintain multiple certifications enabling global distribution:
- FDA Registration: Required for US market access
- CE Certification: European Union compliance
- ISO 13485: International quality management
- GMP Compliance: Good Manufacturing Practice standards
Clean Room Manufacturing Environment
Premium IV set type production requires controlled environments minimizing contamination risks:
- Class 100,000 Clean Rooms: Assembly areas maintaining strict particulate count limits
- Automated Production Lines: Reducing human contact and contamination potential
- Sterile Packaging Systems: Individual pouching ensuring product sterility until use
When Should Healthcare Facilities Upgrade Their IV Set Types?
Technology Assessment Timeline
Healthcare procurement teams should evaluate infusion set technology every 3-5 years, considering:
Safety Incident Analysis: If your facility experiences recurring air embolism incidents or flow-related medication errors, transitioning to double chamber disposable infusion sets should be prioritized immediately.
Regulatory Changes: Updated clinical guidelines or regulatory requirements may necessitate enhanced IV set type specifications. Staying informed about evolving standards ensures compliance and patient safety.
Cost-Benefit Evaluation: While premium infusion sets may carry higher unit costs, reduced complication rates, decreased nursing time for monitoring, and improved patient outcomes often justify the investment.
Shelf Life and Inventory Management
Quality disposable infusion set products typically maintain 5-year shelf life when stored properly, allowing:
- Bulk purchasing advantages without expiration concerns
- Strategic inventory positioning across multiple facilities
- Emergency stockpile maintenance for disaster preparedness
How to Select the Right Infusion Set Specifications for Your Facility
Step 1: Clinical Requirements Assessment
Patient Population Analysis: Pediatric facilities require different IV set types than adult acute care hospitals. Consider:
- Average patient age and size
- Common diagnoses and treatment protocols
- Typical infusion durations
- Required flow rates
Infusion Modality Review: Determine the ratio of gravity versus pressure infusions in your facility. Mixed-use environments benefit from pressure-rated disposable infusion sets providing maximum versatility.
Step 2: Technical Specification Selection
Drip Rate Configuration: Choose between 15 drops/mL (macro-drip) for general adult applications or 20 drops/mL (standard) for precise pediatric dosing. Some facilities maintain both infusion set types for optimal flexibility.
Tubing Length: Select appropriate lengths based on typical patient positioning and equipment placement:
- 1.2m (120cm): Compact option for bedside IV poles
- 1.5m (150cm): Standard configuration for most applications
- 1.8m (180cm): Extended length for specialized positioning needs
Connector Type: Luer slip connectors offer quick attachment, while luer lock configurations provide additional security for high-pressure applications and patient transport scenarios.
Step 3: Material Selection for Special Populations
DEHP-Free Considerations: The American Academy of Pediatrics recommends DEHP-free IV set types for:
- Neonatal intensive care patients
- Pregnant women
- Male infants and adolescent boys
- Patients receiving lipid-based medications
TPE Advanced Materials: Thermoplastic elastomer disposable infusion sets offer:
- Improved flexibility in cold environments
- Reduced kinking and flow obstruction
- Enhanced biocompatibility profiles
- Environmentally friendly disposal characteristics
Step 4: Customization and Branding Options
OEM Manufacturing Services: Leading infusion set manufacturers offer customization including:
- Structural Modifications: Adjusted tubing lengths, specialized connector types, integrated extension sets
- Material Selection: Choice of PVC, DEHP-free, or TPE formulations
- Packaging Customization: Private label branding, language-specific instructions, custom carton configurations
- Component Selection: Specialized filters, multiple injection sites, integrated check valves
Quality Assurance Programs: When partnering with an IV set type manufacturer, establish:
- Incoming inspection protocols
- Performance testing requirements
- Documentation and traceability standards
- Adverse event reporting procedures


How Much Do Quality Infusion Sets Cost? Investment Analysis
Unit Cost Considerations
Disposable infusion set pricing varies based on specifications:
- Standard Single-Chamber Sets: $0.15-$0.35 per unit (bulk wholesale)
- Double Chamber Infusion Sets: $0.25-$0.55 per unit (bulk wholesale)
- Premium DEHP-Free/TPE Sets: $0.40-$0.80 per unit (bulk wholesale)
Note: Prices reflect typical wholesale quantities (100,000+ units). Smaller orders and custom configurations carry premium pricing.
Total Cost of Ownership
Smart procurement teams evaluate IV set type selection beyond unit price:
Complication Cost Avoidance: A single air embolism incident can generate $25,000-$100,000 in additional treatment costs, legal expenses, and regulatory scrutiny. If enhanced infusion set safety features prevent just one incident annually, the investment justifies itself many times over.
Nursing Time Efficiency: Double chamber visibility reduces monitoring frequency requirements. If nurses save just 2 minutes per disposable infusion set insertion through reduced troubleshooting and flow adjustment, multiply those savings across thousands of annual procedures.
Inventory Management: Standardizing on versatile pressure-rated infusion sets eliminates the need to stock multiple specialized types, reducing inventory carrying costs and preventing stock-outs.
Implementation Best Practices: How to Transition Successfully
Staff Training and Education
Introducing new IV set type technology requires comprehensive training:
Hands-On Workshops: Provide opportunities for nursing staff to practice with the new double chamber infusion set design before clinical implementation. Familiarity reduces errors during high-pressure situations.
Competency Validation: Document staff proficiency with the new disposable infusion set through return demonstrations and written assessments.
Reference Materials: Develop quick-reference guides highlighting differences from previous infusion set models, troubleshooting tips, and key safety features.
Phased Implementation Strategy
Pilot Program: Begin with a single department or unit, gathering feedback before facility-wide rollout. This approach identifies workflow issues and refinement opportunities.
Parallel Inventory Management: During transition periods, maintain adequate stock of both old and new IV set types preventing supply disruptions.
Performance Monitoring: Track key metrics including:
- Insertion success rates
- Flow-related troubleshooting calls
- Air-in-line incidents
- Patient satisfaction scores
- Staff feedback and concerns
Quality Assurance: What to Expect from Premium Manufacturers
Testing and Validation Protocols
Reputable infusion set manufacturers conduct extensive quality testing:
Sterility Assurance: Every batch undergoes sterility validation following ISO 11737 standards, ensuring products remain contamination-free throughout their 5-year shelf life.
Flow Rate Accuracy: Precision testing confirms drip chamber performance meets specified rates (15 or 20 drops/mL) within tight tolerances, critical for medication dosing accuracy.
Material Biocompatibility: Comprehensive testing per ISO 10993 standards evaluates cytotoxicity, sensitization, and irritation potential, particularly important for DEHP-free and TPE disposable infusion set formulations.
Pressure Resistance: P-type IV set types undergo rigorous pressure testing ensuring integrity under infusion pump conditions without rupture or leakage risks.
Supply Chain Transparency
Premium manufacturers provide:
Component Traceability: Full documentation of raw material sources, production dates, and quality control checkpoints for every infusion set batch.
Regulatory Documentation: Complete technical files, certificates of conformity, and regulatory submissions supporting import and distribution across global markets.
Audit Access: Factory inspection opportunities allowing distributors and large healthcare systems to verify manufacturing conditions, quality systems, and compliance programs firsthand.



Conclusion: Making the Right Infusion Set Choice for Your Organization
Selecting the optimal infusion set solution requires balancing clinical requirements, patient safety priorities, budget constraints, and operational considerations. The double chamber infusion set represents the current gold standard in intravenous therapy delivery, offering enhanced visualization, auto liquid stop protection, and versatility across clinical applications.
Whether you’re a hospital procurement manager evaluating IV set type options, a medical distributor seeking reliable disposable infusion set suppliers, or a healthcare administrator planning facility upgrades, partnering with an ISO 13485-certified manufacturer ensures access to quality products meeting international regulatory standards.
Key Takeaways:
✅ Double chamber technology provides superior air detection and flow visualization compared to traditional single-chamber designs
✅ Material options including DEHP-free and TPE formulations address special population safety concerns
✅ Dual-rating systems (gravity and pressure) maximize clinical versatility and inventory efficiency
✅ Total cost analysis should consider complication avoidance and efficiency gains, not just unit pricing
✅ Customization capabilities enable OEM partnerships and private label programs for distributors
✅ Quality certifications (ISO 13485, CE marking) serve as essential supplier selection criteria
Next Steps:
For healthcare facilities ready to upgrade their intravenous therapy equipment, request product samples and technical specifications from certified manufacturers. Conduct pilot programs in representative clinical areas before committing to facility-wide transitions.
Medical distributors exploring infusion set product lines should evaluate potential manufacturing partners based on certification credentials, customization flexibility, and supply chain reliability.
Visit www.kohope.com to explore our complete range of double chamber infusion sets, request custom OEM specifications, and connect with our technical support team for application-specific guidance.
Frequently Asked Questions About Infusion Sets
Q: What’s the difference between gravity and pressure infusion sets?
A: Gravity IV set types rely on natural fluid flow driven by height differential between the IV container and insertion site. Pressure infusion sets feature reinforced construction allowing use with infusion pumps generating positive pressure for controlled, rapid delivery. Pressure-rated disposable infusion sets work for both applications, providing maximum versatility.
Q: How often should infusion sets be changed?
A: CDC guidelines recommend changing primary infusion set tubing every 96 hours (4 days) for continuous infusions, or after each use for intermittent infusions. Sets used for blood products or lipid emulsions require more frequent changes. Always follow manufacturer instructions and institutional protocols.
Q: Are DEHP-free infusion sets necessary for all patients?
A: While standard PVC disposable infusion sets are safe for most adult patients, DEHP-free alternatives are recommended for vulnerable populations including neonates, pregnant women, and patients receiving lipid-based medications or long-term therapy. Many facilities adopt DEHP-free IV set types universally to eliminate risk entirely.
Q: Can double chamber infusion sets be used with any IV container?
A: Quality double chamber infusion sets feature universal vented spikes compatible with glass bottles, plastic bags, and semi-rigid containers. Verify compatibility with your specific container types during product evaluation.
Q: What minimum order quantities do manufacturers typically require?
A: Standard infusion set products often have minimum orders of 50,000-100,000 units for wholesale pricing. Custom OEM configurations may require 500,000+ unit commitments. Some manufacturers offer smaller trial quantities at premium pricing for evaluation purposes.

About the Author: This technical guide was developed in consultation with medical device engineering specialists, hospital procurement professionals, and clinical nursing experts to provide comprehensive, accurate information supporting healthcare supply chain decision-making.
Disclaimer: This article provides general information about infusion set technology and selection considerations. Always consult with clinical specialists, regulatory advisors, and qualified medical professionals when making healthcare product procurement decisions. Product specifications and regulatory requirements vary by jurisdiction.