Description
Professional Y Site IV Administration Solutions for Healthcare Facilities
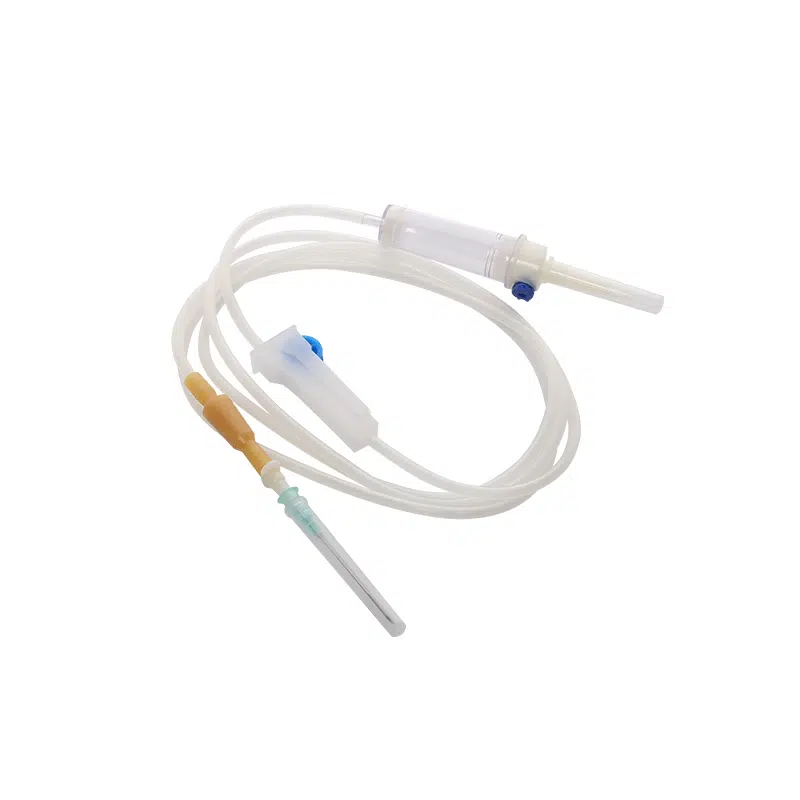
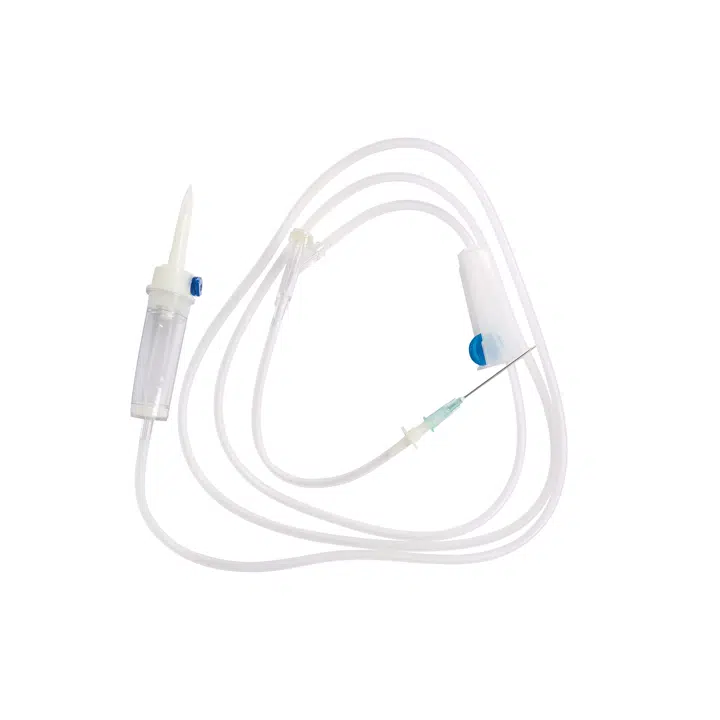
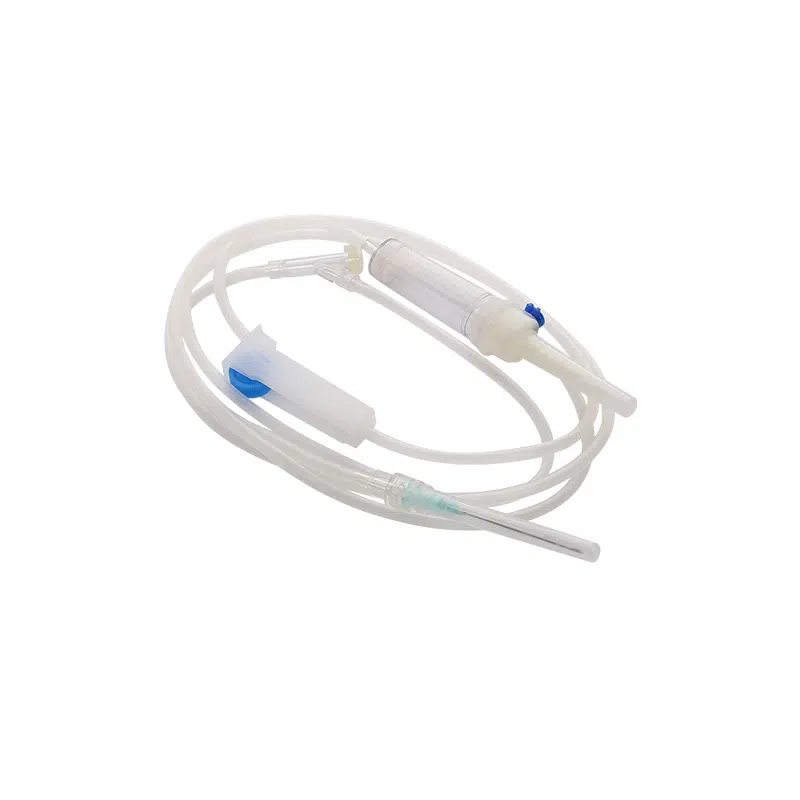
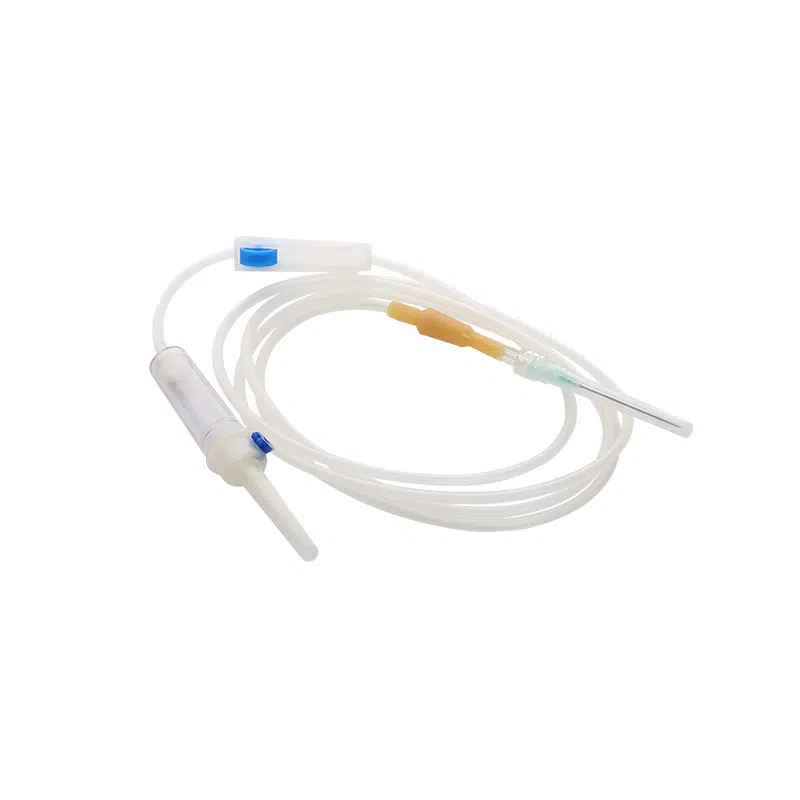
Our Medical Disposable Y Site IV Infusion Set is engineered specifically for healthcare professionals who demand reliability in critical medication delivery scenarios. Understanding that y site iv administration carries inherent risks of drug incompatibility and cross-contamination, we’ve designed this y administration set with optimal safety features to address your key procurement concerns.
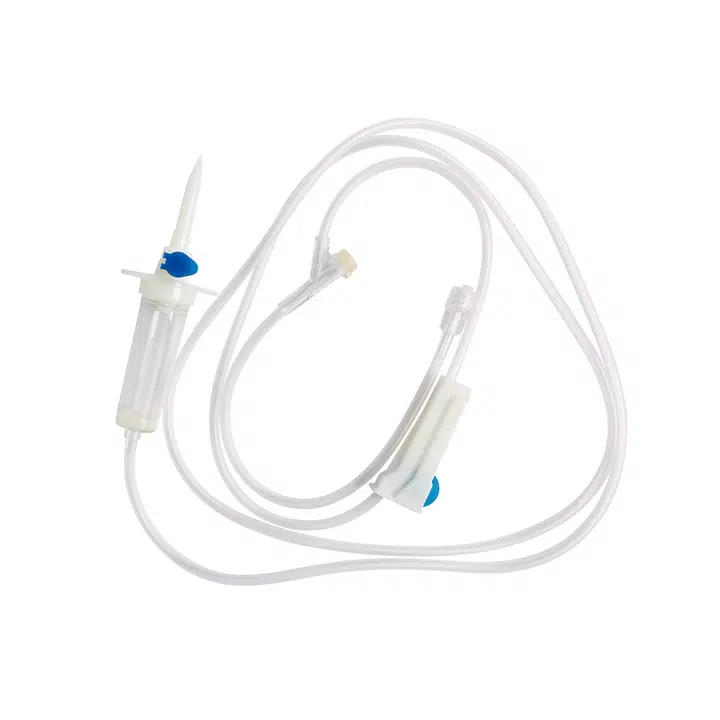
Why Healthcare Buyers Choose Our Y Site IV Sets:
This sterile, single-use infusion system solves the most pressing challenges in simultaneous medication administration. When your clinical teams manage multiple IV therapies—whether piggyback antibiotics during primary fluid infusions or emergency IV push medications—compatibility and contamination prevention become critical. Our y site iv connector design ensures smooth medication mixing while maintaining the integrity of each drug pathway.
Key Procurement Advantages:
- Risk Mitigation: E.O gas sterilization guarantees non-toxic, pyrogen-free delivery, eliminating cross-infection concerns that compromise patient safety and increase liability
- Cost Efficiency: 3-year shelf life reduces inventory waste and storage costs, while our 500,000 PCS/day capacity ensures consistent supply chain stability
- Clinical Versatility: 1.5m length provides flexibility for diverse clinical settings—from ICU multi-drug protocols to pediatric care where precise administration is crucial
- Compliance Ready: Meets international medical device standards, simplifying your regulatory documentation and quality audits
Free Sample Program: Validate product quality and clinical fit before bulk commitment—request your complimentary sample to test compatibility with your facility’s protocols.
Product Details Page
Technical Specifications
Product Name: Medical Disposable Infusion Set with Y Site IV Port
Model: Y Administration Set (Gravity Infusion)
Tubing Length: 1.5m (Standard)
Sterilization: Ethylene Oxide (E.O) Gas
Shelf Life: 3 Years
HS Code: 90183900
Production Capacity: 500,000 pieces/day
Clinical Applications & Use Cases
Primary Applications for Y Site IV Administration:
- Multi-Drug Therapy Management
- Simultaneous primary fluid infusion with secondary medication piggyback (antibiotics, analgesics, chemotherapy agents)
- Emergency department rapid medication delivery while maintaining continuous fluid support
- ICU critical care scenarios requiring multiple vasopressors or sedation protocols
- Pediatric & High-Risk Patient Care
- Precise medication titration where y administration set ports enable controlled bolus delivery
- Neonatal units where fluid volume and drug compatibility monitoring is paramount
- Geriatric care with multiple concurrent medications requiring careful administration timing
- Surgical & Post-Operative Settings
- Anesthesia maintenance with analgesic supplementation via y site iv access
- Post-op pain management protocols combining continuous and intermittent dosing
Addressing Critical Procurement Pain Points
Pain Point 1: Drug Compatibility & Patient Safety
Your Concern: Incompatible medications mixing at the Y-site can cause precipitation, loss of drug efficacy, or toxic reactions—especially with high-alert medications like heparin, phenytoin, or chemotherapy agents.
Our Solution: The Y-site injection port design facilitates proper flushing protocols between incompatible drugs. Transparent tubing allows visual inspection for precipitation. Each set undergoes rigorous quality testing to ensure material compatibility with common medication pH ranges and chemical properties. Included guidelines recommend saline flushes between incompatible medication sequences, reducing adverse events that increase your facility’s litigation risk and regulatory scrutiny.
Pain Point 2: Cross-Contamination & Infection Control
Your Concern: Reusable or inadequately sterilized infusion equipment introduces bloodstream infection risks, driving up hospital-acquired infection rates, patient mortality, and treatment costs.
Our Solution: Single-use disposable design eliminates cross-contamination between patients. E.O gas sterilization achieves medical-grade sterility without toxic residues. Non-pyrogenic certification prevents febrile reactions. PE packaging maintains sterility until point-of-use. This directly supports your infection control initiatives and reduces costs associated with catheter-related bloodstream infections (estimated $25,000+ per incident).
Pain Point 3: Supply Chain Reliability & Inventory Management
Your Concern: Stockouts of critical IV supplies disrupt patient care, while overstocking ties up capital and risks product expiration.
Our Solution: Our 500,000 PCS/day production capacity ensures consistent availability even during demand surges (pandemic scenarios, seasonal illness peaks). The 3-year expiration date allows strategic bulk purchasing with minimal waste—reducing per-unit costs through volume discounts while maintaining financial flexibility. Predictable lead times support just-in-time inventory strategies that optimize your supply chain efficiency.
Pain Point 4: Clinical Staff Training & Medication Errors
Your Concern: Complex infusion equipment increases training time, user error rates, and medication administration mistakes—particularly concerning for y site iv administration where clamping, flushing, and timing errors can have serious consequences.
Our Solution: Intuitive Y-site connector design follows standardized clinical protocols your staff already knows. Clear tubing markings and ergonomic port access reduce confusion during high-pressure situations. The gravity infusion system eliminates electronic pump programming errors. Simplified operation means faster staff onboarding, lower training costs, and fewer medication administration errors that trigger incident reports and regulatory investigations.
Quality Certifications & Compliance
- Sterility Assurance: E.O gas sterilization validated per ISO 11135 standards
- Biocompatibility: Non-toxic, non-pyrogenic materials tested per ISO 10993
- Manufacturing Standards: Produced in ISO 13485 certified facilities with full quality traceability
- Regulatory Compliance: Meets FDA, CE, and international medical device regulations for Class II devices
Packaging & Logistics
Transport Package: Individual PE sterile packaging with tamper-evident seals
Carton Specifications: [Bulk quantity] sets per master carton, optimized for container shipping
Labeling Options: Custom logo printing available for private label partnerships
Documentation: Each shipment includes Certificates of Analysis, Sterilization Validation, and Regulatory Compliance documents
Ordering Information & Support
Minimum Order Quantity: [Contact for current MOQ based on customization requirements]
Sample Policy: Complimentary samples available—cover only shipping costs
Lead Time: Standard orders ship within [X] days; custom printing adds [Y] days
Payment Terms: Flexible terms available for established healthcare distributors and hospital networks
Technical Support: Our medical device specialists assist with:
- Drug compatibility reference charts for common y administration set scenarios
- Clinical protocol development for safe y site iv medication delivery
- Staff training materials and best practice guidelines
- Regulatory documentation support for your facility’s compliance needs

Why Partner With Us
For Hospital Procurement Departments: Reduce total cost of ownership through reliable supply, extended shelf life, and decreased infection-related expenses.
For Medical Distributors: Differentiate your portfolio with high-quality, competitively priced infusion sets backed by responsive customer service and flexible order quantities.
For Group Purchasing Organizations: Volume pricing structures and consistent quality support your members’ cost containment and patient safety goals.
Contact us today for volume pricing, custom specifications, or to request your free evaluation samples. Let us support your mission of safe, efficient patient care delivery.