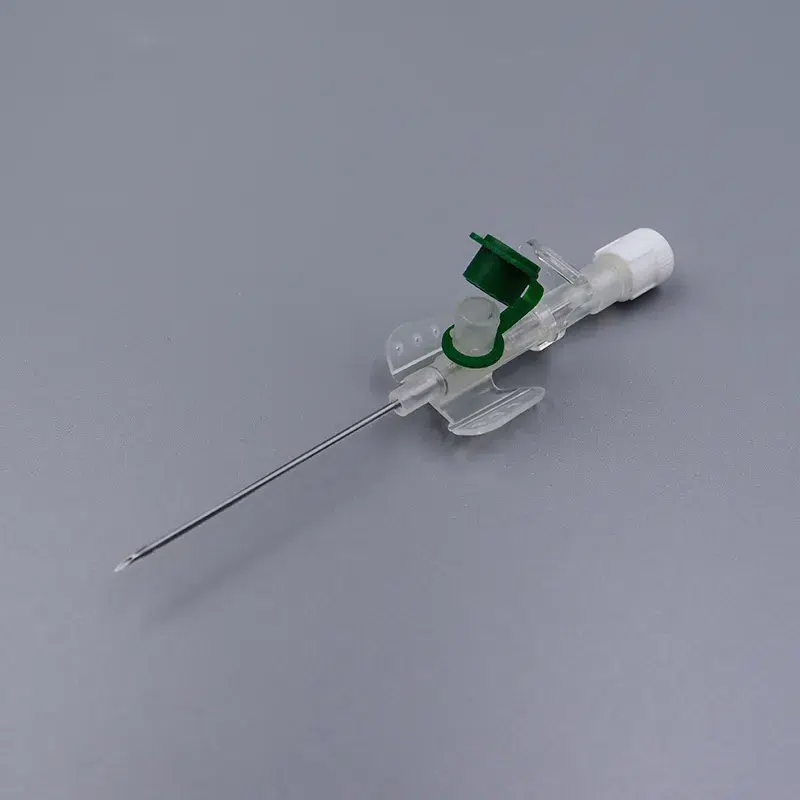
As Singapore’s population aging accelerates and healthcare service demands continue to surge, medical institutions at all levels are imposing increasingly stringent standards for IV cannula procurement. According to Singapore Ministry of Health’s 2024 statistics, public and private hospitals nationwide consume over 8 million venous catheters annually, with improper cannula size selection still resulting in a 12-15% secondary puncture rate. For procurement managers and medical supply chain administrators, balancing iv cannula price control while ensuring product quality has become a critical factor affecting departmental operational efficiency.
This article examines the Singapore medical market’s actual needs in depth, analyzing iv cannula uses (intravenous catheter application scenarios), specification selection standards, pricing composition factors, and how to optimize procurement costs through wholesale and OEM customization.

Part One: Three Major Procurement Pain Points for Singapore Medical Institutions
Pain Point 1: Complex Multi-Specification Inventory Management, High Cannula Size Selection Error Rate
Major comprehensive medical institutions like Singapore General Hospital and Tan Tock Seng Hospital typically need to stock seven IV cannula specifications simultaneously, from 14G to 26G. Emergency departments prefer 18G and 20G for rapid fluid resuscitation, while oncology chemotherapy centers rely more heavily on 22G and 24G fine needles to protect patient vessels. However, many private clinics and community healthcare centers commonly encounter these issues due to lack of professional guidance:
- Over-procurement of large-gauge models: 16G cannula usage rates at non-trauma centers in Singapore remain below 5%, yet occupy 20% of inventory space
- Pediatric-specific specification shortages: KK Women’s and Children’s Hospital reports frequent stockouts of 24G and 26G butterfly models, forcing pediatric nurses to use adult specifications
Solution: Establish dynamic ratio models based on historical consumption data. For example, a 300-bed general hospital should stock 18G:20G:22G:24G in a 3:4:2:1 ratio, potentially reducing expiration losses by 30%.
Pain Point 2: IV Cannula Price Volatility, Lack of Negotiating Power in Bulk Purchases
Singapore’s medical device market heavily depends on imports, with IV cannula pricing affected by three factors: exchange rates, freight, and certification costs. The 2024 SGD-to-USD exchange rate fluctuation caused some European and American brand products to increase 8-12% in cost. For small and medium-sized medical institutions with annual purchases below 500,000 units, the per-unit iv cannula price typically runs SGD 0.15-0.25 higher than large hospitals.
Cost Structure Analysis:
- Factory price: 35-40% of total cost
- International logistics and customs clearance: 20-25% (despite Singapore’s port efficiency, small-batch consolidation costs remain significant)
- HSA certification and compliance: 15-18%
- Distributor markup: 20-25%
Procurement Strategy: Form purchasing alliances with similar medical institutions, or directly engage manufacturers like Kohope for OEM customization, bypassing intermediaries to save 22-30% in costs.
Pain Point 3: Insufficient Understanding of Clinical IV Cannula Uses Requirements
Many procurement personnel focus solely on price and basic parameters, overlooking Singapore’s unique clinical application scenario requirements:
- Dengue fever outbreak surge demands: During the June-October rainy season annually, emergency department IV cannula usage spikes 40%, requiring stockpiling three months in advance
- Fragile vessels in elderly patients: Singapore’s 65+ population comprises 19%, requiring more butterfly models equipped with safety wings and blood flashback viewing chambers
- Multicultural patient-provider communication: Products equipped with Malay and Chinese instruction labels are more popular among community clinics
Part Two: In-Depth Analysis of Cannula Size Selection Standards
Medical Indications and Specification Reference Table
| Cannula Size | Needle OD | Typical IV Cannula Uses | Common Singapore Departments |
|---|---|---|---|
| 14G cannula | 2.0mm | Massive transfusion, trauma resuscitation | NUH Emergency, SGH Trauma Center |
| 16G cannula | 1.7mm | Operating room rapid fluid resuscitation | CGH Anesthesiology |
| 18G cannula | 1.3mm | Standard infusion, antibiotic administration | General wards, outpatient infusion rooms |
| 20G cannula | 1.0mm | Routine hydration, blood collection | Community hospitals, health screening centers |
| 22G cannula | 0.9mm | Pediatrics, geriatrics routine treatment | KK Hospital, Mount Alvernia geriatric wards |
| 24G cannula | 0.7mm | Neonates, chemotherapy patients | KKH NICU |
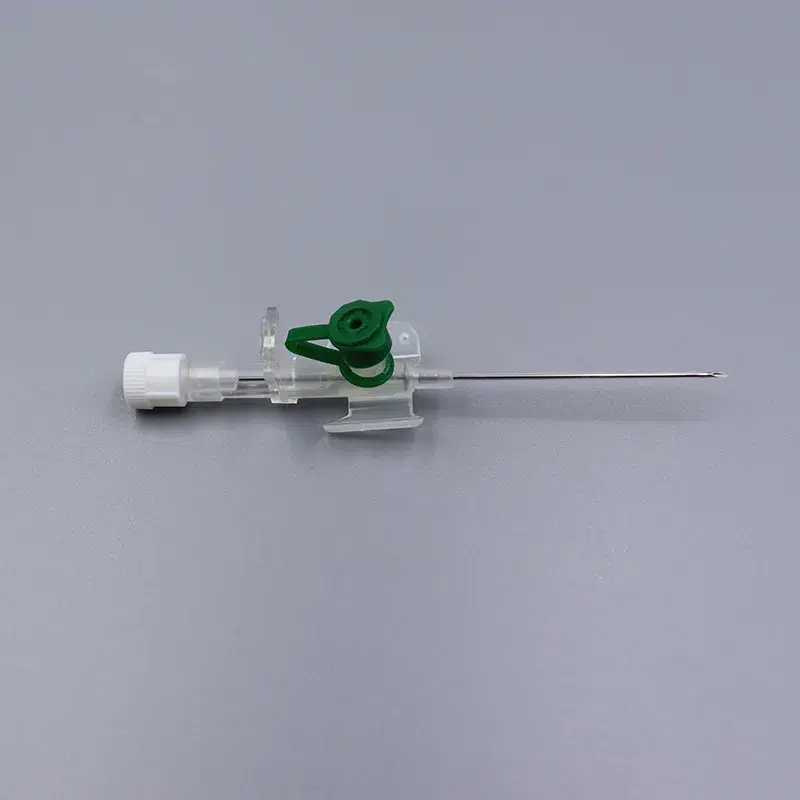
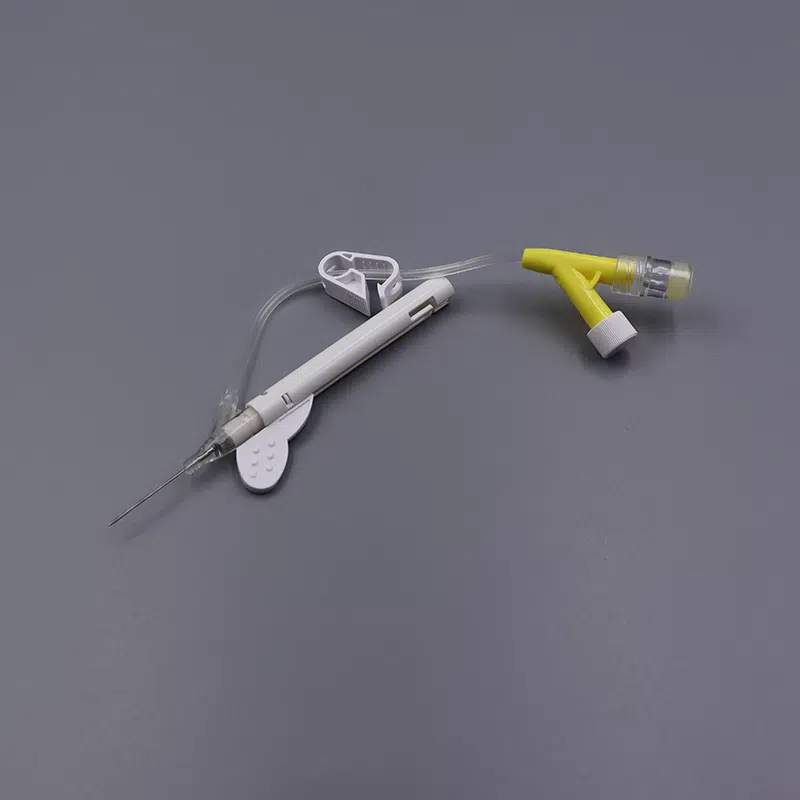
Singapore Medical Institution Specification Preference Data
According to the 2024 Singapore Medical Device Suppliers Association report:
- 18G and 20G account for 62% of total sales, representing public hospital demand mainstays
- 22G demand annual growth rate reaches 18%, driven by population aging
- 14G and 16G comprise only 8%, concentrated primarily in six Level III trauma centers
Selection Recommendations:
- General hospitals: Prioritize stocking three core specifications—18G, 20G, and 22G
- Specialty clinics: Customize according to departmental characteristics (e.g., dialysis centers emphasize 16G, dermatology clinics primarily use 24G)
- Day surgery centers: 20G paired with Y-type injection port design reduces medication change frequency
Part Three: IV Cannula Price Cost Optimization Strategies
Singapore Market Price Benchmarks (2025 Q1 Data)
| Product Type | European/American Brands | Chinese Manufacturers (Kohope, etc.) | Price Difference |
|---|---|---|---|
| Standard 18G | SGD 0.65-0.85 | SGD 0.38-0.52 | Save 40-42% |
| Butterfly 22G | SGD 0.78-0.95 | SGD 0.45-0.60 | Save 37-39% |
| With Injection Port 20G | SGD 0.88-1.10 | SGD 0.52-0.68 | Save 38-41% |
Cost Comparison of Three Procurement Models
Model 1: Traditional Distributor Procurement
- Per-unit iv cannula price: SGD 0.70-0.90
- MOQ requirement: Typically 5,000-10,000 units
- Payment terms: Net 30-60 days
- Suitable for: Small clinics with annual usage <200,000 units
Model 2: Wholesale Direct Purchasing
- Per-unit price: SGD 0.45-0.65 (30% reduction)
- MOQ requirement: Starting from 50,000 units
- Payment terms: Negotiable LC or OA 90 days
- Suitable for: Hospital groups, clinic chains
Model 3: OEM Customization
- Per-unit price: SGD 0.38-0.55 (40%+ reduction)
- MOQ requirement: 100,000 units (mixed cannula size available)
- Value-added services: Custom packaging, multilingual labels, exclusive specification combinations
- Suitable for: Large medical groups, government procurement projects
Case Study: A Singapore 15-location clinic chain switched from distributor procurement to Kohope OEM customization in 2024:
- Annual procurement volume: 1.2 million units (18G/20G/22G mixed ratio)
- Cost savings: SGD 312,000/year
- Additional benefit: Custom packaging featuring clinic logo enhances brand image
Part Four: IV Cannula Uses Clinical Application Scenario Details
Scenario 1: Emergency Department Rapid Venous Access Establishment
Demand Characteristics:
- Time-critical, requiring puncture completion within 5 seconds
- Patients often in shock state with collapsed vessels
- Need simultaneous connection to iv giving set for rapid fluid resuscitation
Recommended Configuration:
- Primary specifications: 18G cannula (70%) + 20G cannula (30%)
- Essential features: Transparent flashback chamber (confirms successful puncture), hydrophobic filter membrane (prevents blood backflow contamination)
- Kohope advantage: Our 18G products use precision-finished PET catheters with 40% improved kink resistance over standard PU materials, preventing twisting during emergency compression procedures
Scenario 2: Oncology Chemotherapy Administration
Demand Characteristics:
- Patients require long-term repeated punctures with poor vascular conditions
- Chemotherapy drugs are highly irritating, requiring stable indwelling
- Frequent medication administration through injection port for anti-nausea drugs and nutritional fluids
Recommended Configuration:
- Primary specifications: 22G cannula (60%) + 20G cannula (40%)
- Essential features: Butterfly wing design (facilitates fixation), Y-type injection port (reduces puncture frequency)
- IV cannula uses extension: When used with PICC catheters, can maintain 7-14 day indwelling time
Singapore-specific needs: National Cancer Centre feedback indicates products with bilingual Chinese-English medication labels reduce patient-provider communication costs by 25%.
Scenario 3: Pediatric and Neonatal Care
Demand Characteristics:
- Vessel diameter only 1-2mm, requiring 24G/26G ultra-fine needles
- Non-cooperative pediatric patients demand first-attempt puncture success
- Parents highly concerned about product safety and pain levels
Recommended Configuration:
- Primary specifications: 24G cannula (80%) + 22G cannula (20%)
- Essential features: Short needle length design (19mm), smooth needle tip (reduces tissue damage)
- Kohope innovation: Our 24G products use triple-facet needle tip grinding technology, reducing puncture resistance by 35%; KK Hospital pediatrics testing shows first-attempt success rates improved to 92%
Scenario 4: Geriatric Ward Chronic Disease Management
Demand Characteristics:
- Patients have fragile vessels prone to hematoma formation
- Require long-term indwelling (48-72 hours)
- Often combined with diabetes and other underlying conditions, high infection risk
Recommended Configuration:
- Primary specifications: 20G cannula (50%) + 22G cannula (50%)
- Essential features: Soft PU catheter (conforms to vessel walls), transparent dressing observation window (early detection of leakage)
- Cannula size selection principle: Prioritize smallest gauge meeting infusion rate requirements to reduce mechanical phlebitis incidence
Part Five: Singapore Procurement Processes and Compliance Essentials
HSA Registration Requirements
All IV cannula products entering the Singapore market must comply with:
- Medical Devices Act: Class B medical device registration (typically requires 3-6 months)
- ISO 13485 certification: Quality management system documentation
- Biocompatibility testing: ISO 10993 standards
- Sterility assurance: EO sterilization validation reports
Kohope Certification Advantages:
- HSA certification obtained (registration number available upon request)
- CE/FDA dual certification, qualifying for Singapore’s “secondary certification fast track”
- Provides complete CoC (Certificate of Conformity) and sterilization batch records
Government Procurement Bidding Essentials
Singapore Ministry of Health publishes public hospital centralized procurement requirements through the GeBIZ platform, with key scoring criteria:
- Price competitiveness (40% weight): Requires detailed iv cannula price breakdown table
- Technical specifications (30% weight): Product performance test reports, comprehensive cannula size coverage capability
- Supply stability (20% weight): Production capacity proof (Kohope monthly capacity: 5 million units), Singapore local warehousing
- After-sales service (10% weight): 7-day response, product training support
Winning Tips:
- Provide “basic + premium” combination solutions (e.g., standard model + injection port model)
- Commit to 15% backup inventory to address dengue fever and other outbreak emergencies
- Include complimentary clinical usage training (CPD credit courses recognized by Singapore Nursing Academy)

Part Six: Why Choose Kohope as Your IV Cannula Supplier
Six Advantages Tailored for the Singapore Market
Advantage 1: Flexible Cannula Size Combination Solutions
- Support mixed-specification minimum orders (freely combine 14G-26G within 50,000 units)
- Based on Singapore’s seasonal demand fluctuations, provide “summer emergency packages” (18G+20G primary) and “standard ward packages” (20G+22G primary)
Advantage 2: Highly Competitive IV Cannula Price
- Annual procurement volumes exceeding 1 million units receive additional 3-5% discounts
- Transparent pricing structure with no hidden fees
Advantage 3: Deep Understanding of Clinical IV Cannula Uses Requirements
- Technical team has served Singapore General Hospital, Mount Elizabeth Hospital, and other institutions
- Can customize according to departmental characteristics (e.g., nephrology dialysis-specific reinforced 16G)
- Provides trilingual product instructions in Chinese, English, and Malay
Advantage 4: Rapid Singapore Logistics Response
- Direct shipping from Guangzhou/Shenzhen ports, 7-10 days sea freight to Singapore
- Partnership with PSA port customs clearance agents, 2-day customs completion
- Experienced in handling Singapore’s stringent import documentation requirements
Advantage 5: OEM Customization Meets Branding Needs
- Minimum 100,000 units for custom packaging (printing clinic logos, contact information)
- Develop exclusive cannula size ratios (e.g., “community clinic standard package”: 20G 60% + 22G 30% + 24G 10%)
- Provide product photography and promotional material design support
Advantage 6: Comprehensive Quality Traceability System
- Each batch assigned unique LOT number, traceable to raw material suppliers
- Committed 0.08% low defect rate (industry average 0.15%)
- 7-day no-questions-asked return policy (within Singapore)

Part Seven: How to Begin Cooperation with Kohope (Action Guide)
Step 1: Assess Your Actual Needs (Free Requirement Analysis)
Contact Kohope’s Singapore business team; we’ll assist you with:
- Analyzing past 12 months’ IV cannula consumption data
- Identifying high-frequency cannula size usage and inefficient inventory
- Calculating annual savings from switching to Kohope supply
Required Materials:
- Monthly average usage statistics for each specification
- Current supplier quotation sheets
- Special clinical requirement descriptions (e.g., need for injection port, butterfly wings, etc.)
Step 2: Request Sample Testing (48-Hour Dispatch)
We provide complimentary sample packages covering:
- 5 core cannula size options (20 units each of 18G/20G/22G/24G/26G)
- 3 product types (standard/butterfly/Y-injection port models)
- Complete testing guide (puncture resistance, flashback speed, catheter flexibility evaluation standards)
Testing Period: Recommend 2-4 weeks clinical trial, collecting nursing team feedback
Step 3: Customize Your Procurement Solution
Based on testing results, Kohope provides three comparative solutions:
- Solution A (Economy): Pure standard models, lowest iv cannula price
- Solution B (Balanced): 80% standard + 20% premium models (with injection port)
- Solution C (Premium): Full butterfly models + custom packaging, suitable for private medical brands
Each solution includes:
- Detailed cannula size ratio tables
- 5-year total cost of ownership (TCO) analysis
- Logistics timeline and inventory turnover recommendations
Step 4: Sign Contract and Arrange Production
First-Order Benefits:
- Waive OEM design fees for orders below 100,000 units (valued at SGD 3,500)
- Complimentary first delivery shipping within Singapore
- Provide 3-month payment terms (Net 90)
Production Cycles:
- Standard model in-stock: 7-10 day delivery
- OEM customization: 25-30 day delivery
- Rush orders: Expedited production coordination available (advance communication required)
Step 5: Continuous Optimization and Support
After cooperation commences, Kohope provides:
- Quarterly product usage training (Singapore Nursing Academy CPD certified)
- Annual iv cannula uses clinical symposiums (inviting local KOLs to share insights)
- Dedicated account manager (response time <4 hours)
- Regular product performance reviews and specification optimization recommendations
About Kohope
Kohope Medical is an ISO 13485-certified enterprise specializing in intravenous catheter R&D and manufacturing for 15 years. Our products are exported to 65 countries, serving over 3,000 medical institutions. We understand the high-standard requirements of Singapore’s healthcare system and are committed to providing “European/American quality, competitive pricing” solutions as your trustworthy long-term partner.
For detailed product specifications, current pricing, and sample requests, visit our IV cannula product page or contact our Singapore business development team directly.