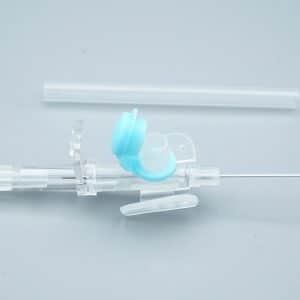
Description
Why Healthcare Providers Choose Kohope IV Cannula Solutions
Where clinical accuracy meets patient comfort, our butterfly IV cannula systems excel in emergency departments, surgical units, oncology centers, and pediatric wards across the USA, Canada, and European markets.

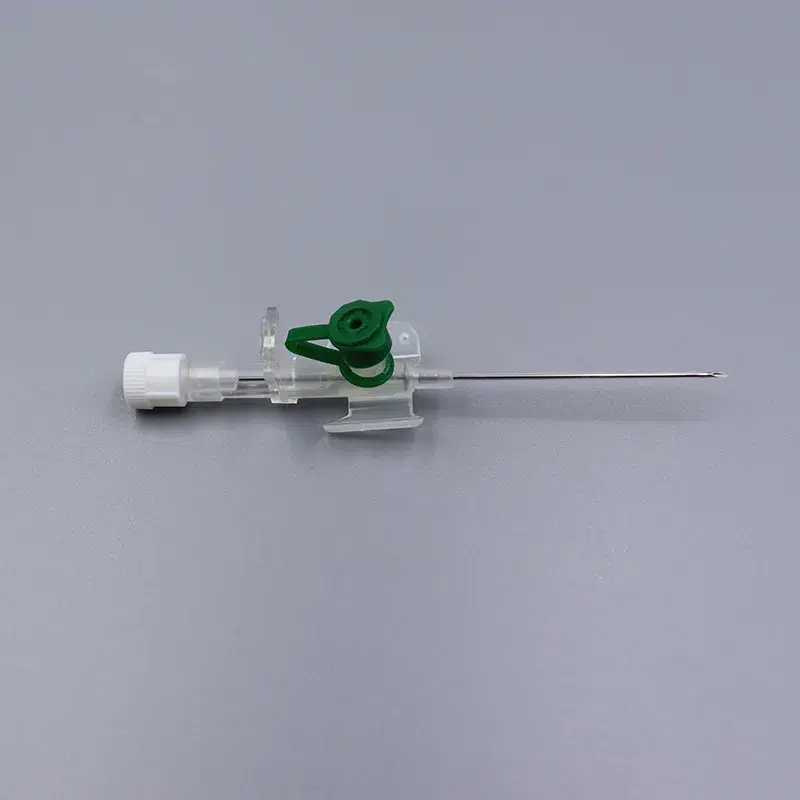
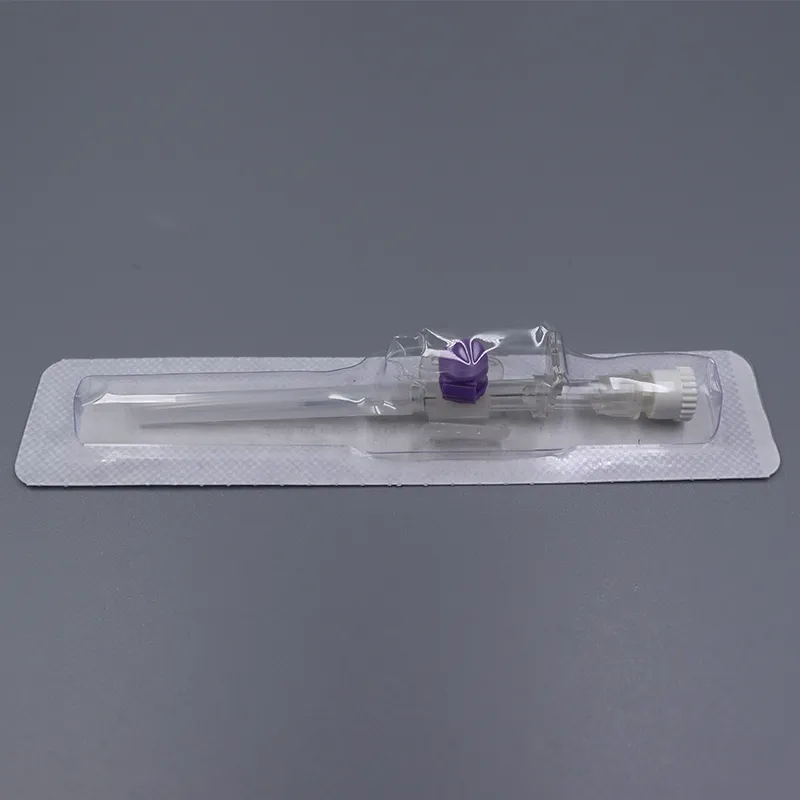
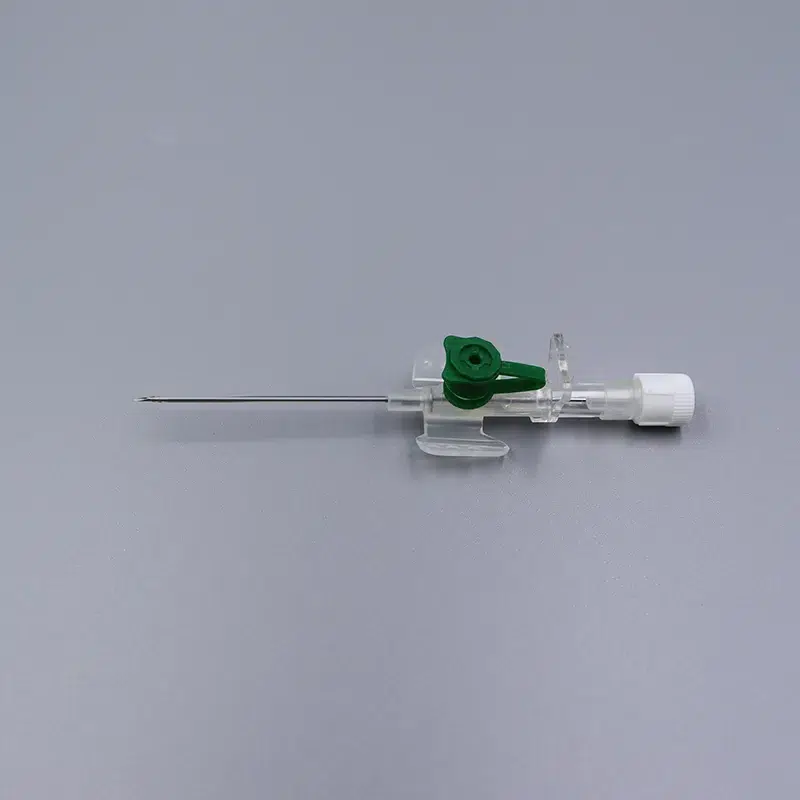
What makes our product essential: Each 18g cannula, 20g cannula, and 22g cannula incorporates precision-finished PET/PU catheters that prevent kink and ensure stable flow during critical infusions. The integrated injection port eliminates repeated needle sticks—a vital feature when nurses administer medication through the IV giving set without disconnecting the primary line.
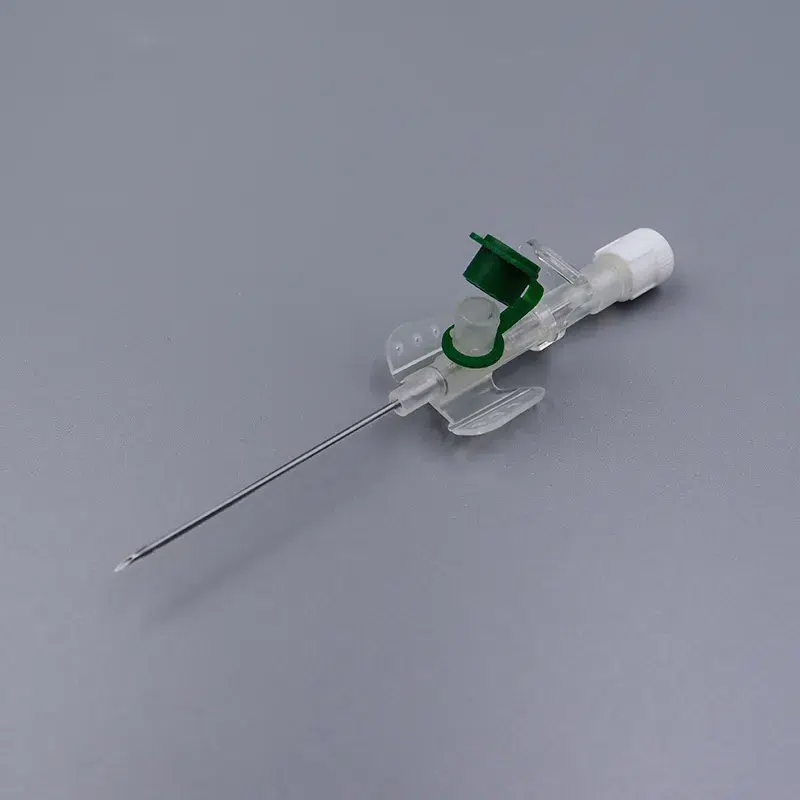
Who benefits from bulk purchasing: Hospital procurement teams reducing supply costs, medical distributors expanding catheter portfolios, and ambulance service operators requiring reliable venous access tools. Our 14g cannula and 16g cannula options serve trauma cases demanding rapid fluid resuscitation, while the 24g cannula suits neonatal and geriatric patients with fragile veins.
When speed matters in emergencies, color-coded hubs (orange for 14g cannula, gray for 16g cannula, green for 18g cannula) enable instant size recognition under pressure. Translucent flashback chambers confirm successful venipuncture within seconds—critical in shock management and anesthesia induction.

How our OEM program works: Kohope provides custom needle lengths (19mm–45mm), tailored packaging with your branding, and gauge assortments matching your regional demand patterns. Whether you need 10,000 units of 22g cannula for outpatient clinics or mixed pallets combining 16g cannula through 24g cannula for hospital networks, our production scales to your specifications.
Manufacturing advantages for wholesale partners: Hydrophobic membrane filters prevent backflow contamination—a key differentiator when competing for hospital contracts. Our pen-type, winged-type, butterfly-type, and Y-type configurations offer solutions for every clinical scenario, from standard ward use to specialized oncology IV giving set protocols.
Certification & Compliance: ISO 13485 medical device quality systems, CE marked for EU distribution, and FDA-registered facility. HS Code 9018321000 ensures smooth customs clearance for international shipments.
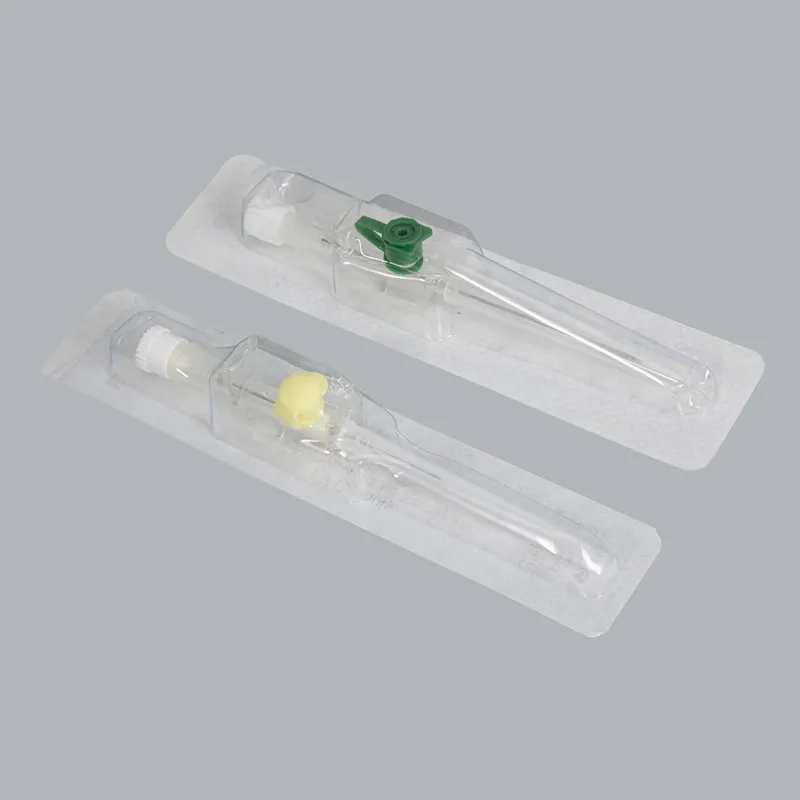
Order with confidence: Sample kits available to evaluate 18g cannula, 20g cannula, and 22g cannula performance in your target markets. Volume discounts start at 50,000 units. Lead time: 25–35 days for OEM orders, 7–15 days for stock configurations.
Contact Kohope today to discuss your IV cannula wholesale requirements and custom branding opportunities.