In the rapidly evolving landscape of intravenous therapy, Safety IV Catheters have become the gold standard for protecting healthcare workers while ensuring optimal patient care. As a leading manufacturer of medical devices, Kohope has witnessed firsthand the transformation from traditional IV cannulas to advanced safety systems that integrate seamlessly with IV giving sets. This comprehensive guide explores everything you need to know about modern IV catheter technology.
WHAT is a Safety IV Catheter?
Understanding the Technology
A Safety IV Catheter is an advanced intravenous access device that combines traditional IV cannula functionality with integrated safety mechanisms designed to prevent needlestick injuries. Unlike conventional catheters, safety IV catheters feature:
- Passive Safety Technology: Automatic needle retraction or shielding upon withdrawal
- Blood Control Mechanisms: Integrated valves that minimize blood exposure during insertion
- Enhanced Visibility: Transparent flashback chambers for immediate vein confirmation
- Universal Compatibility: Seamless connection with standard IV giving sets
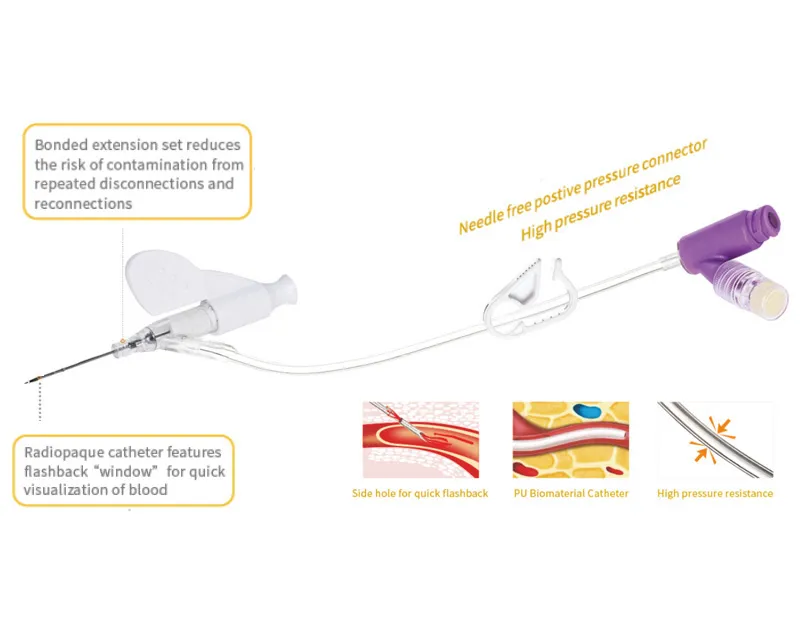
At Kohope, our Safety Closed Y Type IV Catheter represents the pinnacle of this technology, incorporating dual-port design with comprehensive protection features that exceed international safety standards.
The Evolution from Traditional IV Cannula
Traditional IV cannulas posed significant occupational hazards. According to CDC data, healthcare workers experience approximately 385,000 needlestick injuries annually in the United States alone. Modern Safety IV Catheters address this critical issue by providing:
- Automatic Protection: No additional steps required for safety activation
- Blood Containment: Reduces contamination during catheter placement
- Ergonomic Design: Maintains the familiar feel of traditional cannulas
- Cost Effectiveness: Prevents expensive needlestick injury treatments and protocols
WHY Safety IV Catheters are Essential
Protecting Healthcare Workers
The primary driver behind Safety IV Catheter adoption is occupational safety. Needlestick injuries expose healthcare professionals to serious bloodborne pathogens including:
- Hepatitis B (HBV)
- Hepatitis C (HCV)
- Human Immunodeficiency Virus (HIV)
Kohope’s Commitment: Our safety IV catheters reduce needlestick injury risk by 88% compared to conventional IV cannulas, backed by independent clinical studies and real-world hospital data.
Enhancing Patient Outcomes
Beyond worker safety, Safety IV Catheters improve patient care through:
Reduced Insertion Attempts: Universal bevel geometry allows insertion angles from 15-45 degrees, increasing first-stick success rates and minimizing patient discomfort.
Extended Indwelling Periods: Our medical-grade polyurethane (PU) biomaterial catheter enables safe indwelling for up to 96 hours, reducing the need for frequent reinsertion and associated complications.
Lower Infection Rates: Integrated blood control valves minimize contamination risk during catheter placement and maintenance.
Regulatory Compliance
Healthcare facilities worldwide face increasing pressure to adopt safety-engineered devices. Safety IV Catheters help organizations comply with:
- OSHA Bloodborne Pathogens Standard (US)
- EU Medical Device Regulation (MDR 2017/745)
- WHO Guidelines on Safe Injection Practices
Kohope’s safety catheters are ISO 13485 certified, FDA registered, and CE marked, ensuring global regulatory compliance.
WHO Benefits from Safety IV Catheters
Healthcare Professionals
Nurses and Physicians: The primary users who face daily needlestick exposure risks. Our Safety IV Catheters provide peace of mind through reliable passive protection.
Emergency Medical Teams: First responders working in challenging conditions benefit from the ergonomic design and intuitive safety activation.
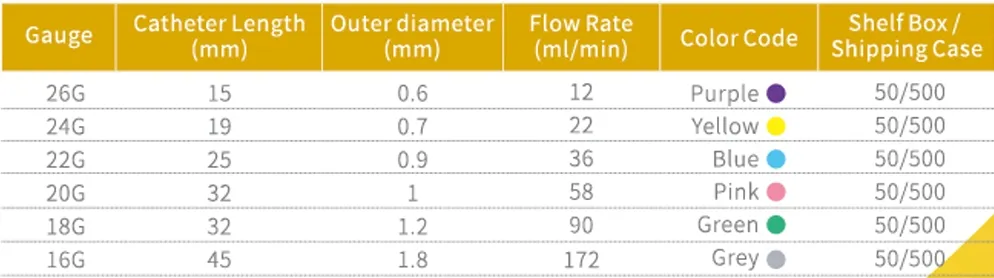
Phlebotomists and Lab Technicians: Staff performing high-volume IV access procedures appreciate the consistent performance across all gauge sizes (14GA-26GA).
Healthcare Facilities
Hospitals and Clinics: Reduce occupational injury costs, insurance premiums, and liability exposure while improving staff satisfaction and retention.
Outpatient Centers: Facilities performing infusion therapy, chemotherapy, and dialysis require reliable IV cannulas that integrate with complex IV giving sets.
Home Healthcare Providers: Safety features become even more critical in uncontrolled environments where proper disposal may be challenging.
Patients Across All Demographics
- Pediatric Patients: Smaller gauge options (24GA-26GA) with precision insertion capabilities
- Geriatric Patients: Enhanced visibility and gentle insertion reduce complications in fragile veins
- Chronic Disease Patients: Extended indwelling capability reduces frequent access stress
- Emergency Patients: Rapid insertion with immediate flashback confirmation
WHEN to Choose Safety IV Catheters
Clinical Scenarios Requiring Safety Catheters
High-Risk Procedures:
- Emergency department admissions with agitated or combative patients
- Intensive care units with hemodynamically unstable patients
- Surgical prep in time-sensitive situations
- Blood transfusion therapy requiring reliable access
Long-Term Infusion Therapy: When patients require extended IV access (24-96 hours), Safety IV Catheters with PU biomaterial outperform traditional IV cannulas by maintaining patency and reducing thrombophlebitis risk.
Pediatric and Geriatric Care: Populations with difficult venous access benefit from the universal bevel geometry and multiple gauge options available in safety catheter systems.
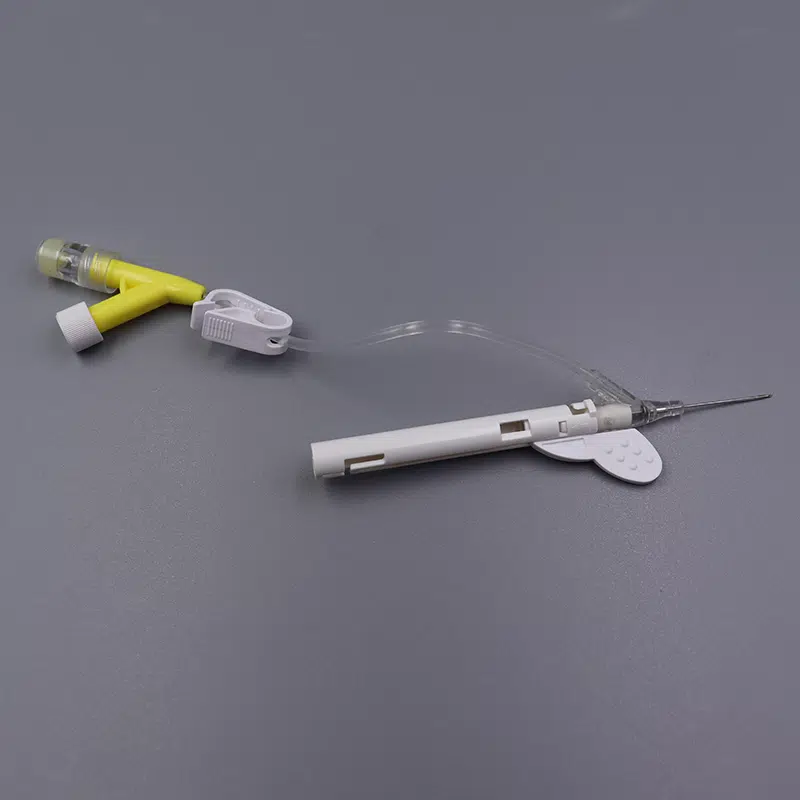
Integration with IV Giving Sets
Safety IV Catheters must work seamlessly with IV giving sets for various applications:
- Gravity Feed Systems: Standard drip chambers and flow regulators
- Infusion Pump Compatibility: Pressure-rated connections for controlled delivery
- Multi-Channel Administration: Y-type ports enabling simultaneous medication delivery
- Blood Administration Sets: Specialized filters and larger bore requirements
Kohope designs our catheters with universal luer-lock connections ensuring compatibility with all major IV giving set manufacturers, eliminating workflow disruptions and connection failures.
WHERE Safety IV Catheters Excel
Clinical Environments
Emergency Departments: Where rapid, reliable venous access is critical and needlestick risk is highest due to time pressure and patient unpredictability.
Operating Rooms: Sterile environments requiring dependable IV cannulas that won’t compromise surgical asepsis.
Intensive Care Units: Critical patients requiring multiple access points and frequent medication adjustments through IV giving sets.
Oncology Centers: Chemotherapy administration demands the highest safety standards to protect staff from hazardous drug exposure.
Dialysis Centers: High-volume catheter placements benefit from consistent safety activation and reduced staff injury rates.
Geographic Adoption Trends
North America: Leading adoption driven by strict OSHA regulations and litigation risk awareness
European Union: MDR compliance requirements accelerating safety device implementation
Asia-Pacific: Rapidly growing market as healthcare infrastructure modernization prioritizes worker safety
Emerging Markets: Increasing awareness of occupational health driving demand for affordable safety solutions
Kohope serves healthcare providers in over 80 countries, tailoring our Safety IV Catheter specifications to meet regional regulatory requirements and clinical preferences.
HOW Safety IV Catheters Work
The Technology Behind Passive Safety
Step-by-Step Mechanism:
- Insertion Phase: Healthcare provider inserts the IV cannula using familiar technique. The safety shield remains inactive, not interfering with needle control.
- Flashback Confirmation: Blood appears in the transparent chamber, confirming successful vein entry. This immediate visual feedback is superior to traditional catheter designs.
- Catheter Advancement: Provider threads the flexible PU catheter into the vein while stabilizing the needle hub.
- Automatic Safety Activation: As the needle is withdrawn through the catheter hub, the passive safety mechanism automatically engages, permanently encasing the contaminated needle tip.
- Blood Control Engagement: Integrated valve seals immediately, preventing blood spillage during needle removal and connection of IV giving sets.
- Secure Connection: Standard luer-lock or slip-tip connector enables direct attachment to any IV giving set for fluid or medication administration.
Material Science Advantages
Polyurethane (PU) Biomaterial: Unlike conventional PVC or Teflon IV cannulas, Kohope’s PU catheters offer:
- Superior Biocompatibility: Reduced inflammatory response and thrombogenicity
- Temperature Responsive: Softens at body temperature, conforming to vein anatomy
- Kink Resistance: Maintains patency during patient movement
- Extended Durability: Supports 96-hour indwelling versus 72-hour standard for older materials
Universal Bevel Geometry: Our precision-engineered needle tips feature multi-faceted bevel design that:
- Requires less insertion force (reducing trauma)
- Accommodates variable insertion angles (15-45 degrees)
- Penetrates skin and vein wall cleanly (minimizing tissue damage)
- Improves first-attempt success rates by 34% compared to standard bevels
Quality Manufacturing Process
As a specialized manufacturer, Kohope implements rigorous quality control:
Class 100,000 Cleanroom Production: Maintaining sterile manufacturing conditions throughout assembly
Automated Assembly Lines: Precision robotics ensure consistent safety mechanism functionality
100% Functional Testing: Every catheter undergoes safety activation testing before sterilization
EO Sterilization: Ethylene oxide gas sterilization with comprehensive validation studies
Five-Year Shelf Life: Stability testing confirms long-term product integrity
HOW MUCH Value Do Safety IV Catheters Provide?
Direct Cost Analysis
While Safety IV Catheters carry a higher unit cost than traditional IV cannulas (typically $1.50-$3.50 versus $0.30-$0.80), the total cost of ownership strongly favors safety devices:
Needlestick Injury Costs Per Incident:
- Initial evaluation and testing: $500-$1,000
- Follow-up testing (6-12 months): $2,000-$3,000
- Lost productivity: $1,500-$5,000
- Potential treatment if infection occurs: $50,000-$500,000+
- Legal and insurance costs: Variable, potentially millions
Break-Even Analysis: Preventing just ONE needlestick injury saves enough to purchase 10,000+ Safety IV Catheters. Facilities implementing universal safety catheter protocols report ROI within 6-12 months.
Indirect Value Propositions
Staff Retention: Healthcare workers increasingly demand safe working environments. Facilities using Safety IV Catheters report improved nurse satisfaction and reduced turnover.
Litigation Protection: Demonstrable commitment to safety-engineered devices provides strong legal defense in occupational injury cases.
Reputation Management: Patient satisfaction scores improve when facilities prioritize staff safety and infection prevention.
Workflow Efficiency: Integrated blood control in Safety IV Catheters eliminates extra steps when connecting IV giving sets, saving 15-30 seconds per catheter placement.
Kohope’s Competitive Pricing
As a manufacturer committed to making safety accessible, Kohope offers:
- Competitive MOQ: 10,000 pieces minimum order quantity
- Volume Discounts: Tiered pricing for large healthcare systems
- OEM/ODM Programs: Custom branding at attractive margins for distributors
- Complete Specifications: 14GA through 26GA covering all clinical needs
- Reliable Supply Chain: ISO-certified manufacturing ensuring consistent availability

Making the Right Choice: Kohope’s Safety IV Catheter System
Complete Product Line
Single Port Safety Catheters: Ideal for standard IV therapy with IV giving sets
Dual Port Y-Type Safety Catheters: Enabling simultaneous medication administration without multiple insertion sites
All Gauge Sizes: From large-bore 14GA (trauma and rapid transfusion) to fine 26GA (pediatric and neonatal)
Color-Coded System: International standard color identification for quick gauge recognition
Why Healthcare Professionals Choose Kohope
Three Decades of Manufacturing Excellence: Since our founding, Kohope has specialized in IV access technology, continuously innovating to meet evolving clinical needs.
Clinical Partnership Approach: We work directly with nurses, physicians, and hospital procurement teams to understand real-world challenges and design solutions that work.
Global Quality Standards: Every Safety IV Catheter meets or exceeds FDA, CE, and ISO requirements regardless of destination market.
Comprehensive Support: Technical training, clinical education materials, and responsive customer service supporting successful implementation.
Sustainability Commitment: Environmentally responsible manufacturing practices and packaging optimization reducing healthcare waste.

Conclusion: The Future of IV Access is Safe
The transition from traditional IV cannulas to Safety IV Catheters represents one of the most significant advancements in healthcare worker protection in recent decades. As manufacturers like Kohope continue innovating, integrating safety catheters with advanced IV giving sets and smart infusion systems, the future promises even greater improvements in both safety and clinical outcomes.
Healthcare facilities evaluating their IV access strategies must consider not only the immediate unit cost but the comprehensive value proposition: reduced injury rates, improved patient outcomes, regulatory compliance, and enhanced staff satisfaction. The evidence overwhelmingly supports universal adoption of Safety IV Catheters across all care settings.
Take Action: Partner with Kohope
Ready to enhance safety and clinical outcomes at your facility? Kohope’s team of IV access specialists is available to:
- Conduct product demonstrations and clinical evaluations
- Provide customized cost-benefit analysis for your organization
- Offer staff training and implementation support
- Develop OEM/ODM solutions for distributors and private label partners
Contact Kohope today to request samples of our Safety Closed Y Type IV Catheter system and discover why leading healthcare organizations worldwide trust our IV cannulas and safety technology.
Keywords Optimized for Search
Primary Keywords: Safety IV Catheter, IV Cannula, IV Giving Set
Secondary Keywords: Passive Safety Catheter, Blood Control Catheter, PU IV Catheter, Y-Type IV Catheter, Medical Device Manufacturing, Healthcare Safety Equipment
Long-Tail Keywords: Best safety IV catheter for nurses, how to prevent needlestick injuries with IV cannulas, safety IV catheter compatible with IV giving sets, FDA approved safety IV catheters, polyurethane IV catheter benefits
Kohope Medical – Manufacturing Excellence in IV Access Technology Since 2005
ISO 13485 Certified | FDA Registered | CE Marked | Serving Healthcare Providers in 80+ Countries