Advancing Diabetic Care in Veterinary Medicine Through Innovation and Precision
Pet diabetes management has emerged as one of the most significant health challenges in modern veterinary practice, affecting millions of companion animals worldwide. As the prevalence of diabetes continues to rise among our beloved pets, veterinary insulin syringes have become indispensable veterinary medical devices, revolutionizing how we approach diabetic cat dog treatment and animal insulin injection protocols. These precision-engineered, single-use insulin syringes provide safe, accurate insulin delivery while offering unprecedented convenience and reliability for both veterinary professionals and pet owners seeking effective pet diabetes management solutions.
Understanding Veterinary Insulin Syringes: Definition and Clinical Significance
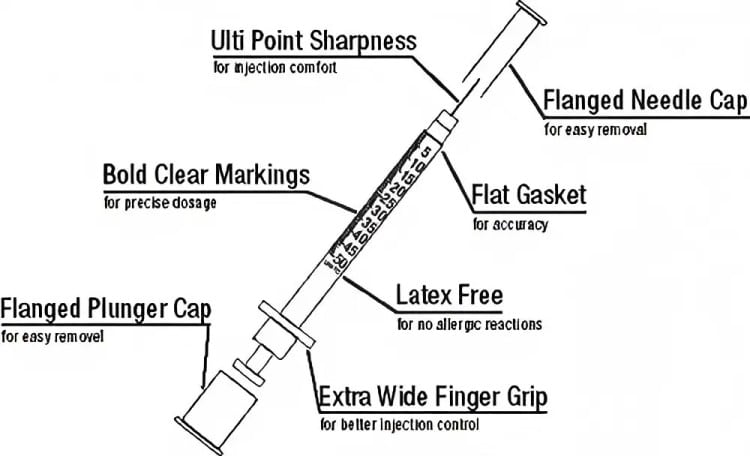
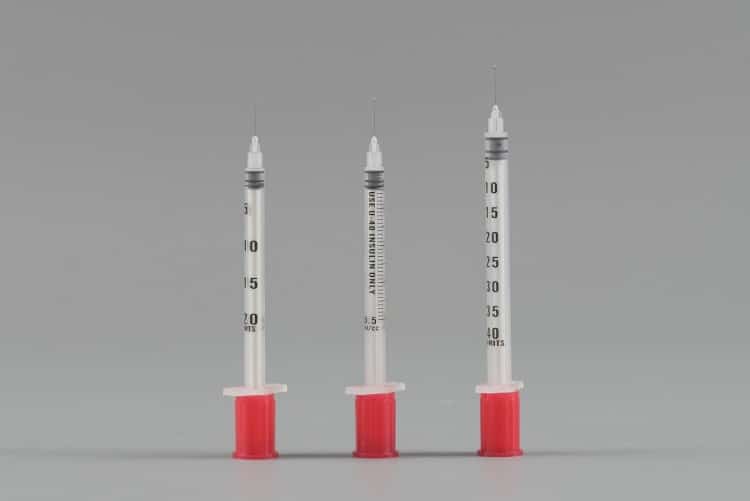
Veterinary insulin syringes represent specialized, single-use insulin syringes specifically engineered for animal insulin injection and pet diabetes management. Unlike their human medical counterparts, these professional-grade veterinary medical devices feature unique design characteristics and technical specifications tailored to meet the diverse needs of different animal species. Manufactured from high-grade medical plastics, these veterinary insulin syringes comprise three critical components: the barrel, plunger, and ultra-fine needle—each meticulously engineered to deliver optimal performance in veterinary applications.
Current veterinary epidemiology indicates that diabetes mellitus affects approximately 1% of the canine population and 0.5% of felines, with these numbers showing a concerning upward trend. For affected animals, regular animal insulin injection represents the cornerstone of life-sustaining therapy and quality of life maintenance. Veterinary insulin syringes provide the essential technical foundation for this critical diabetic cat dog treatment protocol.
The clinical significance of these specialized veterinary medical devices extends far beyond their primary medical function, encompassing animal welfare protection through their thoughtful design. By utilizing purpose-built veterinary insulin syringes, clinicians can significantly reduce injection-associated pain and stress responses, thereby enhancing overall pet diabetes management outcomes and improving patient quality of life.
Safety Excellence and Sterility Assurance in Veterinary Practice
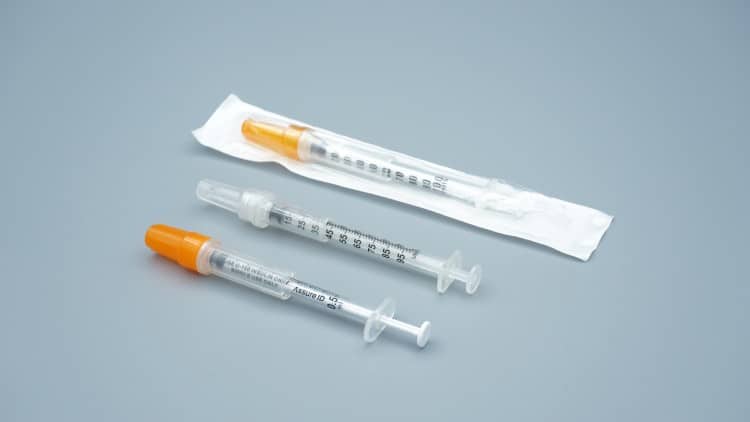
Safety represents the paramount advantage of veterinary insulin syringes. Each single-use insulin syringe undergoes manufacturing in rigorously controlled, sterile environments using internationally standardized sterilization protocols, ensuring complete sterility upon packaging. This pre-sterilization design eliminates cross-contamination risks—particularly critical for diabetic animals whose immune systems may be compromised during pet diabetes management.
In busy veterinary clinic environments where multiple patients receive sequential treatment, single-use insulin syringes completely eliminate bloodborne pathogen transmission risks between animals. This protection extends to veterinary staff while providing additional safety margins. Traditional reusable syringes require complex cleaning and sterilization procedures that, despite strict protocol adherence, still carry inherent contamination risks. Single-use insulin syringes fundamentally eliminate these concerns.
Furthermore, veterinary insulin syringes incorporate multiple safety features. Needles receive silicone treatment for smooth surfaces, reducing tissue trauma and injection site inflammation. Transparent barrel construction enables clear visualization of medication status and dosage accuracy. These comprehensive safety characteristics provide complete protection for diabetic cat dog treatment.
Precision Dosing Control: Core Technical Advantages
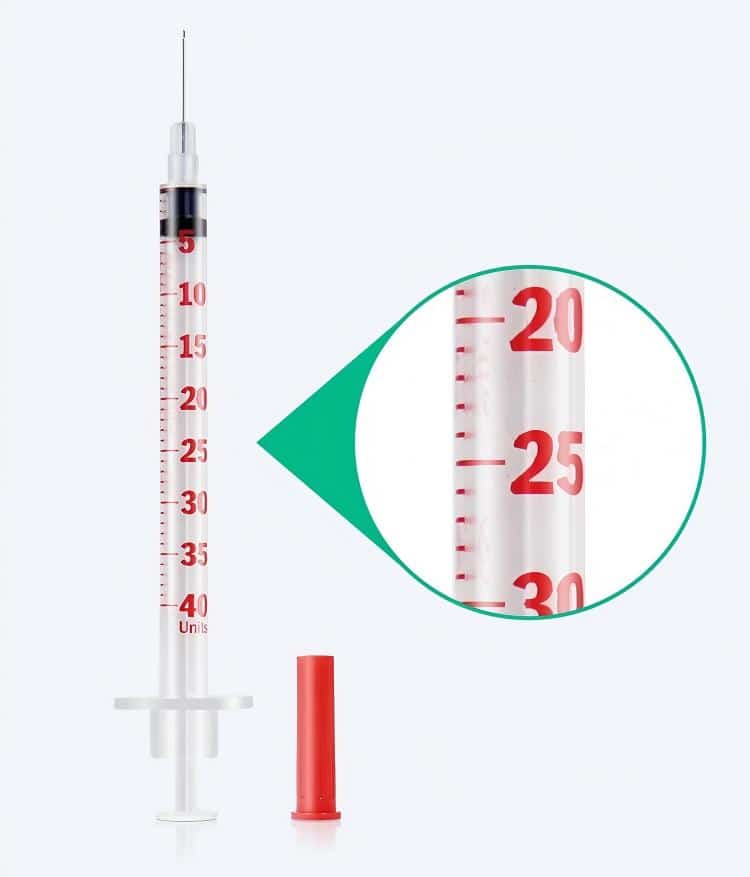
Dosing precision stands as the critical success factor in pet diabetes management. Veterinary insulin syringes demonstrate exceptional technical advantages in this domain. These veterinary medical devices are specifically calibrated for U-40 insulin concentrations commonly used in veterinary practice, distinctly different from the U-100 insulin prevalent in human medicine. This specialized design ensures dosing calculation accuracy while preventing concentration-related medication errors in animal insulin injection procedures.
The graduated measurement systems undergo precise calibration, typically providing 0.1-unit accuracy. For smaller animals like cats requiring minimal insulin doses during diabetic cat dog treatment, this high-precision graduation system proves invaluable. Each graduation mark utilizes laser-etching technology, ensuring long-term clarity and wear resistance, thereby maintaining consistent and accurate dose reading throughout the pet diabetes management process.
Multiple veterinary insulin syringe specifications accommodate various animal sizes. The 0.3mL specification suits small cats and puppies, delivering up to 12 units of U-40 insulin; the 0.5mL specification represents the most commonly used size for medium-sized dogs and cats, providing 20-unit capacity; the 1mL specification accommodates large breed dogs with up to 40-unit insulin capacity. This diverse specification range ensures optimal veterinary medical device matching for every animal’s pet diabetes management needs.
Convenience-Focused Design: Streamlining Diabetic Care Protocols
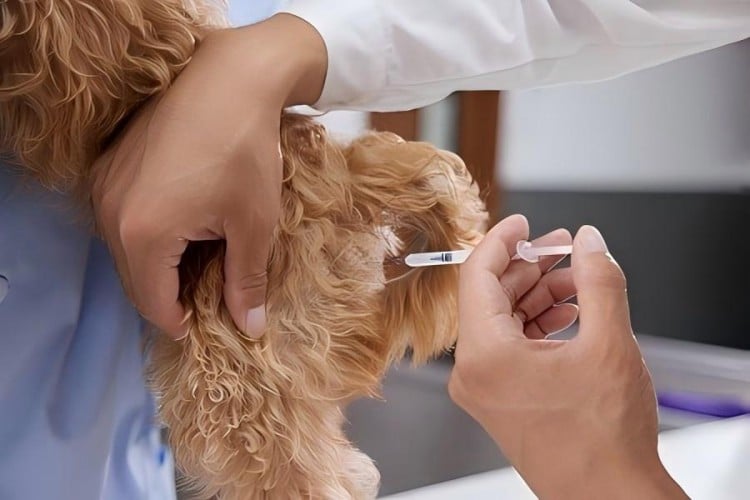
The convenience-oriented design of veterinary insulin syringes manifests throughout every aspect of the treatment process. For veterinary professionals, the ready-to-use, disposable characteristics significantly streamline clinical workflows. Eliminating complex cleaning, sterilization, and maintenance procedures allows medical staff to focus greater time and energy on animal diagnosis and treatment, thereby improving overall medical service quality and efficiency.
In high-volume veterinary practices, time equals life. Single-use veterinary insulin syringes achieve readiness within seconds, dramatically reducing treatment preparation time. This proves especially critical for emergency diabetic ketoacidosis treatment, where rapid medication administration may directly impact animal survival.
For pet owners managing at-home insulin injections, convenience becomes paramount. Many pet owners experience anxiety when first confronting insulin administration. The user-friendly design of veterinary insulin syringes effectively reduces operational complexity. Clear graduation markings, smooth plunger action, and effortless needle insertion enable non-professionals to quickly master proper techniques.
Packaging design also prioritizes convenience. Each syringe features individual sealed packaging for easy storage and transport. For pet owners requiring travel, this portable design ensures treatment continuity regardless of location changes.
Ultra-Fine Needle Technology: Minimizing Pain and Stress
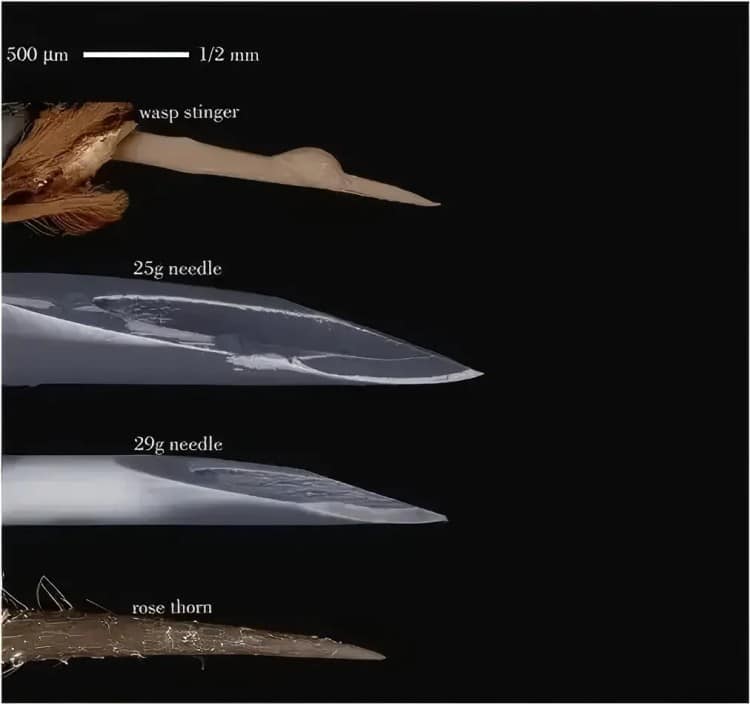
Pain management represents a crucial component of modern veterinary medicine. Veterinary insulin syringes incorporate cutting-edge ultra-fine needle technology, with diameters typically ranging from 29-31 gauge—significantly finer than traditional syringe needles. This ultra-fine design substantially reduces skin penetration discomfort, proving particularly meaningful for diabetic animals requiring multiple daily injections.
Needle length receives careful engineering, typically ranging from 5-8 millimeters. This length ensures accurate subcutaneous insulin delivery while avoiding unnecessary muscle tissue stimulation. Different animal sizes can utilize appropriately sized needles, ensuring both injection accuracy and comfort.
Surface treatment employs advanced silicone technology, creating extremely smooth needle surfaces that reduce skin tissue friction while further minimizing penetration pain. This treatment also reduces tissue trauma, promoting rapid healing at injection sites and decreasing infection risks.
For stress-prone animals, pain reduction helps decrease overall stress responses. Research demonstrates that pain and stress negatively impact blood glucose control, making veterinary insulin syringes indirectly beneficial for overall diabetes management through pain reduction.
Professional Veterinary Applications: Clinical Scenarios

In modern veterinary clinics, veterinary insulin syringe applications span diverse scenarios. Emergency departments frequently manage diabetic ketoacidosis cases requiring rapid, accurate insulin administration. Single-use veterinary insulin syringes achieve immediate readiness, providing precious time for life-saving interventions.
In hospitalization units managing multiple diabetic animals requiring scheduled insulin injections, single-use syringes ensure sterile treatment devices for each patient while significantly simplifying nursing workflows. Staff can focus on monitoring patient condition changes and treatment responses rather than equipment cleaning and maintenance.
Surgical blood glucose management represents another critical application area for veterinary insulin syringes. Surgical stress significantly impacts blood glucose levels, requiring precise insulin adjustments. In sterile operating environments, pre-sterilized single-use syringes ensure no contamination risk to the surgical field.
Specialty clinics, particularly endocrinology practices, routinely provide detailed home care instruction to clients. The standardized design of veterinary insulin syringes simplifies client education effectiveness. Veterinarians can demonstrate using identical equipment that clients use at home, preventing confusion from equipment variations.
Pet Owner Home Care Guidelines

For most diabetic pets, daily insulin therapy occurs in home settings, requiring pet owners to master proper injection techniques and equipment usage. The user-friendly design of veterinary insulin syringes significantly simplifies this learning process.
Regarding storage, veterinary insulin syringes should be kept in dry, cool environments away from direct sunlight. Each package displays expiration dates that require careful pre-use verification. Expired syringes may compromise injection accuracy and present safety hazards.
Pre-use preparation includes insulin status verification, dosage confirmation, and appropriate reward preparation. Insulin typically requires gentle mixing without vigorous agitation to prevent medication structure damage. Allowing insulin to reach room temperature helps reduce injection discomfort.
Injection site selection and rotation prove crucial for long-term therapy. Common injection sites include the scruff, chest wall, and lateral thigh areas. Regular site rotation prevents localized tissue hardening, ensuring consistent insulin absorption. Each injection site should receive alcohol swab cleaning before injection to maintain local cleanliness.
Injection technique mastery requires practice. Proper technique involves rapid needle insertion at 45-90 degree angles, slow plunger advancement, brief post-injection holding before needle withdrawal. Throughout the process, maintaining animal calm and cooperation through appropriate rewards and reassurance helps establish positive treatment experiences.
Technical Specifications and Quality Standards Analysis

Veterinary insulin syringe technical specifications reflect modern medical device manufacturing precision. Syringe barrels typically utilize medical-grade polypropylene with excellent chemical stability and biocompatibility. Transparency exceeds 95%, ensuring clear medication status and dosage graduation visibility.
Graduation system manufacturing employs advanced laser-etching technology, with each graduation line controlled to 0.1-millimeter width and 0.05-millimeter depth, ensuring long-term wear resistance. Numerical markings use medical-grade ink printing with superior chemical corrosion resistance and UV stability.
The plunger system represents a core syringe component. High-quality veterinary insulin syringes employ three-stage sealing designs ensuring advancement seal integrity and smoothness. Plunger heads utilize specialized rubber formulations providing excellent sealing while maintaining smooth sliding performance. Advancement force typically ranges from 2-5 Newtons, ensuring operational ease while preventing accidental rapid advancement.
Needle manufacturing utilizes premium stainless steel, typically 316L medical-grade stainless steel with excellent corrosion resistance and mechanical strength. Manufacturing precision reaches micrometer levels, with outer diameter tolerances within ±0.01 millimeters. Needle tips utilize laser cutting technology ensuring sharpness and consistency.
Quality control requires each batch to undergo rigorous testing procedures including seal integrity testing, dosage precision testing, needle sharpness testing, and biocompatibility testing. Only products passing all tests enter market circulation.
Economic Benefits and Cost-Effectiveness Analysis
While single-use veterinary insulin syringes may present higher per-use costs compared to reusable alternatives, comprehensive economic analysis reveals superior overall cost-effectiveness. This economic advantage manifests through direct cost savings and indirect benefit improvements.
Direct cost savings eliminate cleaning, sterilization, and maintenance expenses. Traditional reusable syringes require specialized cleaning equipment, sterilants, and labor time—costs that accumulate significantly over extended use. Additionally, avoiding cross-contamination risks reduces medical liability and legal risks.
For veterinary clinic operations, single-use syringes simplify inventory management and equipment maintenance workflows. Eliminating expensive autoclaving equipment investments and specialized storage and maintenance space requirements particularly benefits small and medium-sized veterinary practices by significantly reducing operational costs and management complexity.
Indirect benefits include improved treatment efficiency and client satisfaction. Faster treatment preparation enables serving more clients, increasing clinic revenue. Enhanced treatment experiences and safety assurance strengthen client trust, fostering long-term client relationships.
For pet owners, while per-use costs are slightly higher, considering convenience, safety, and improved treatment outcomes, the overall value proposition remains highly attractive. Particularly for busy pet owners, saved time and effort costs often exceed equipment cost differences.
Environmental Responsibility and Sustainability Considerations

As single-use medical devices, the environmental impact of veterinary insulin syringes requires serious consideration. Responsible manufacturers and users must actively minimize negative environmental impacts while advancing sustainability goals.
In product design phases, progressive veterinary insulin syringe manufacturers have begun adopting biodegradable materials and eco-friendly production processes. Some companies are developing plant fiber-based bioplastics replacing traditional petroleum-based plastics, maintaining medical-grade performance while enabling natural environment degradation.
Packaging material eco-friendly development represents another important direction. Adopting recyclable paper packaging replacing plastic packaging, reducing packaging material usage, and utilizing eco-friendly printing inks all constitute effective environmental measures. Some manufacturers offer bulk packaging options, reducing overall packaging waste through decreased individual packaging usage.
During usage, proper waste disposal proves crucial. Veterinary insulin syringes constitute medical waste requiring strict medical waste disposal protocol collection and processing. Never dispose of used syringes in regular garbage to prevent environmental and public safety threats.
Many regions have established medical waste recycling networks providing convenient waste disposal services for veterinary clinics and pet owners. Some manufacturers offer take-back programs where clients can return used syringes to manufacturers for professional processing and resource recovery.
Innovation Technology and Future Development Trends
Technological innovation in veterinary insulin syringes accelerates rapidly, with exciting new technologies and product features emerging over the coming years. These innovations will not only improve product performance but further enhance animal treatment experiences and owner convenience.
Smart functionality represents the most compelling development direction. Next-generation veterinary insulin syringes may integrate micro-sensors and digital display systems, automatically recording injection time, dosage, and injection site information. This data can transmit via Bluetooth or WiFi to smartphone applications, helping pet owners and veterinarians better monitor treatment progress.
Dosage safety locking technology also develops rapidly. This technology can preset specific dosage ranges, preventing accidental overdosing. For anxious or inexperienced pet owners, this safety feature provides important value.
In needle technology, researchers explore more advanced pain reduction solutions including vibration-assisted injection, topical anesthetic coatings, and ultrasound guidance—all currently in testing phases. These technologies will further reduce animal pain and stress responses.
Material science advances bring new possibilities for veterinary insulin syringes. Smart materials can change color based on temperature changes, alerting users to insulin storage status; antimicrobial materials can further reduce infection risks; bioabsorbable materials may completely resolve medical waste disposal issues.
Selection Guidelines and Quality Identification Points
Choosing appropriate veterinary insulin syringes requires considering multiple factors including animal characteristics, usage environment, quality standards, and budget considerations. Professional selection guidelines help veterinarians and pet owners make optimal choices.
First consideration involves animal size and species characteristics. Small cats typically require 0.3mL specification syringes with 29-31G ultra-fine needles; medium-sized dogs and cats suit 0.5mL specifications; large breed dogs may require 1mL specification syringes. Needle length selection also proves important—excessive length may cause unnecessary pain while insufficient length may affect medication absorption.
Insulin type matching represents another key factor. Ensure selected syringe graduation systems match used insulin concentrations. Veterinary practice typically uses U-40 concentration insulin, requiring syringes specifically calibrated for U-40 to avoid dosage calculation errors.
Quality certification serves as an important product reliability indicator. High-quality veterinary insulin syringes should obtain relevant medical device certifications such as FDA, CE, or China NMPA certification. These certifications ensure products meet strict safety and performance standards.
Package integrity also represents an important quality judgment aspect. Each syringe should have complete sealed packaging clearly marking production date, expiration date, and specification information. Damaged or expired products must never be used.
Manufacturer reputation and after-sales service merit consideration. Choosing reputable manufacturers typically provides more reliable product quality and comprehensive after-sales support. Some manufacturers also provide technical training and usage guidance services, particularly valuable for first-time users.
Operational Training and Safety Protocols
Proper operational training provides the foundation for safe and effective veterinary insulin syringe usage. Whether professional veterinary personnel or pet owners, all require systematic training to master correct operational methods and safety protocols.
Basic training content should include equipment structural recognition, dosage calculation methods, injection techniques, and safety disposal procedures. For veterinary professionals, training should also cover emergency situation handling, adverse reaction identification, and client education techniques.
Practical operation training represents an important training component. Under professional guidance, trainees must repeatedly practice dosage drawing, injection techniques, and equipment disposal. Only through sufficient practical training can operational accuracy and safety in actual applications be ensured.
Safety protocol establishment and adherence prove crucial for preventing accidents. This includes personal protective equipment usage, sterile operation procedure execution, and emergency situation response measures. Particularly for pet owners, emphasizing child safety protection and accidental puncture handling methods proves essential.
Regular retraining and skill assessment help maintain operational levels and safety awareness. Annual operational skill reviews are recommended to ensure operational method correctness and safety protocol execution.
Clinical Outcome Evaluation and Treatment Optimization
Clinical outcome evaluation of veterinary insulin syringes represents an important component of diabetes treatment management. Through systematic outcome monitoring and data analysis, treatment protocols can be continuously optimized to improve treatment effectiveness.
Blood glucose monitoring serves as the core indicator for evaluating treatment outcomes. Regular blood glucose testing reflects insulin therapy effectiveness and dosage appropriateness. Modern blood glucose monitoring technology has matured significantly, enabling pet owners to conduct simple blood glucose testing at home, providing timely data support for treatment adjustments.
Body weight changes and appetite status also represent important observation indicators. Diabetic animals under effective treatment should demonstrate stable or moderate weight gain with normal appetite. Persistent weight loss or appetite suppression may indicate treatment protocol adjustments are needed.
Quality of life assessment includes activity capacity, mental state, and behavioral performance. Effective diabetes treatment should restore normal life vitality and behavioral patterns in animals. Persistent lethargy, reduced activity, or abnormal behavior may indicate poor blood glucose control.
Regular veterinary examinations and laboratory testing represent important components of treatment monitoring. This includes blood glucose curve testing, glycated hemoglobin measurement, and other relevant biochemical indicator examinations. These professional tests provide more accurate treatment outcome evaluation and guide treatment adjustments.
Regulatory Standards and Compliance Requirements
As specialized medical devices, veterinary insulin syringes must strictly comply with relevant industry standards and regulatory requirements. These standards not only ensure product quality and safety but also provide standardized guidance for industry development.
International Organization for Standardization (ISO) 7886 standard serves as the fundamental standard for single-use syringes, covering material requirements, design specifications, performance testing, and labeling requirements. For veterinary insulin syringes, compliance with ISO 8537 regarding insulin syringe-specific requirements is also necessary, including graduation precision, dosage accuracy, and needle specifications.
The U.S. Food and Drug Administration (FDA) classifies veterinary insulin syringes as Class II medical devices, requiring 510(k) premarket notification procedures. Manufacturers must demonstrate substantial equivalence to already-approved similar products, including safety and effectiveness evidence. This certification process typically requires 6-12 months involving extensive technical documentation and clinical data submission.
European Union Medical Device Regulation (MDR) implements stricter regulatory requirements for veterinary insulin syringes. Manufacturers must establish complete quality management systems, undergo conformity assessment by notified bodies, and obtain CE marking before European market sales. This process addresses not only product safety and performance but also requires manufacturers to establish comprehensive post-market surveillance systems.
China’s National Medical Products Administration (NMPA) implements classified management for veterinary insulin syringes, with most products listed as Class II medical devices. Manufacturers must pass product registration approval and obtain medical device registration certificates before production and sales. The registration process includes technical review, quality management system inspection, and product testing.
Global Market Analysis and Development Trends
The veterinary insulin syringe market experiences rapid growth, primarily driven by rising pet diabetes incidence rates, improved pet healthcare awareness, and advancing diagnostic technology. Market research data indicates the global veterinary insulin syringe market size is projected to maintain annual growth rates exceeding 15% over the next five years.
North America represents the largest veterinary insulin syringe market, accounting for over 40% of global market share. This primarily results from the region’s advanced pet healthcare systems, higher pet healthcare spending levels, and comprehensive regulatory environments. The United States houses approximately 90 million pet dogs and 94 million pet cats, with diabetes affecting roughly 1-2%, providing substantial market demand for veterinary insulin syringes.
Europe follows closely, accounting for approximately 30% of global market share. European Union countries commonly establish comprehensive animal welfare legal systems with high pet healthcare quality requirements, driving demand growth for high-quality veterinary insulin syringes. Major countries including Germany, the United Kingdom, and France show continuous pet population growth, providing solid market development foundations.
The Asia-Pacific region represents the fastest-growing market with annual growth rates exceeding 20%. Countries including China, Japan, and South Korea experience rapid pet market development with continuously rising pet healthcare spending levels. Particularly in China, urbanization processes and improved living standards drive dramatic pet population growth with vigorous pet healthcare demand.
Manufacturing Processes and Quality Control Systems

Veterinary insulin syringe manufacturing represents highly precise and strictly controlled processes. Entire production must occur in cleanrooms meeting ISO 14644-1 standards, typically requiring Class 8 (ISO 8) or higher cleanliness levels.
Raw material control represents the first step in quality assurance. Polypropylene materials for syringe barrels must comply with USP Class VI biocompatibility standards with excellent chemical stability and low extractable content. Stainless steel materials for needles typically utilize 316L medical-grade steel requiring strict compositional analysis and mechanical property testing.
Injection molding processes represent critical barrel manufacturing components. Modern injection equipment employs precision temperature and pressure control systems ensuring dimensional accuracy and surface quality for each product. Mold design utilizes CAD/CAM technology with graduation lines created through laser-etching processes achieving ±0.05-millimeter precision.
Needle manufacturing employs multiple precision processing procedures. Initial cold drawing processes achieve precise outer diameters and wall thickness, followed by precision cutting for required lengths, and final laser cutting and polishing for sharp, smooth needle tips. Entire processes maintain micrometer-level dimensional tolerances.
Assembly processes utilize automated production lines, reducing manual contact and contamination risks. Each assembly station features inline inspection equipment for real-time product quality monitoring. Critical parameters including seal integrity, dosage precision, and needle sharpness undergo 100% automated inspection.
Sterilization processes typically employ ethylene oxide (EO) or gamma radiation sterilization. EO sterilization suits temperature-sensitive materials, effectively eliminating all microorganisms without affecting product performance. Gamma radiation sterilization offers strong penetration and thorough sterilization, suitable for batch processing.
Packaging processes employ heat-sealing technology ensuring individual product seal integrity. Packaging materials typically utilize medical-grade Tyvek and PET film composite materials with excellent bacterial barrier properties and breathability. Each package undergoes seal integrity inspection ensuring sterile product maintenance throughout shelf life.
Clinical Research and Evidence-Based Medicine

Clinical application effectiveness of veterinary insulin syringes has been validated through extensive scientific research. These studies not only demonstrate product safety and effectiveness but also provide important guidance for clinical applications.
A multicenter randomized controlled trial involving 500 diabetic dogs showed the treatment group using specialized veterinary insulin syringes significantly outperformed the control group using general syringes in blood glucose control effectiveness. The treatment group achieved 23% average blood glucose reduction with 31% decreased glucose fluctuation amplitude—differences proving statistically significant (P<0.01).
Pain assessment research employed standardized animal pain scoring systems comparing different needle gauge impacts on injection pain. Results showed 29G ultra-fine needles reduced pain scores by 47% compared to 25G standard needles while significantly reducing injection site inflammation. This study provided strong scientific evidence for ultra-fine needle technology applications.
Long-term safety studies tracked 300 diabetic pets using veterinary insulin syringes over 2-year observation periods. Results showed infection rates of 0.3% for single-use veterinary insulin syringes, far below the 2.1% infection rate for reusable syringes. This difference primarily resulted from sterile assurance and cross-contamination avoidance of single-use products.
Cost-effectiveness analysis studies evaluated veterinary insulin syringe value from socioeconomic perspectives. While per-use costs were slightly higher, considering reduced infection treatment costs, improved treatment outcomes, and enhanced quality of life, overall cost-effectiveness ratios reached 3.2:1, demonstrating excellent economic value.
User Experience Optimization and Ergonomic Design
Modern veterinary insulin syringe design fully considers ergonomic principles, striving to provide optimal user experiences. This user-centered design philosophy not only improves operational convenience but also enhances treatment safety and effectiveness.
Grip comfort represents an important design consideration. Syringe barrels employ ergonomically conforming designs with diameters typically ranging from 12-15 millimeters—convenient for gripping while avoiding excessive thickness affecting fine operations. Surfaces receive light texturing treatment to increase friction and prevent slippage.
Graduation readability optimization utilizes multiple technical approaches. Graduation lines employ black laser-etching creating strong contrast with transparent barrels, ensuring clear reading under various lighting conditions. Number fonts utilize specialized medical fonts with optimized character spacing and sizing suitable for different age user visual requirements.
Plunger advancement feel calibration represents a technical challenge. Ideal advancement force should range from 2-5 Newtons—too little risks misoperation while too much creates operational difficulty. Through precision sealing ring design and lubrication treatment, smooth and consistent advancement feel is achieved while maintaining constant advancement resistance, avoiding sudden resistance changes.
Package easy-opening design considers different user needs. Packages employ tear-strip designs enabling tool-free easy opening. Tear line design ensures opening processes won’t damage internal products while providing smooth tearing edges to prevent user injury.
Visual design employs clean, contemporary modern styling. Product and package color schemes consider medical environment characteristics, typically utilizing blue-white color schemes that appear both professional and approachable. Important information receives color coding and icon design highlighting for rapid identification.
Specialized Applications and Customized Solutions
Veterinary insulin syringes in certain specialized applications require customized solutions. These special needs drive product innovation and technological advancement while opening new industry development directions.
Wildlife rescue scenarios impose special requirements on veterinary insulin syringes. Wild animals typically exhibit strong stress responses requiring rapid, accurate medication administration. Specialized long-needle versions enable safe distance injection while maintaining adequate strength and sharpness—needle lengths may reach 25 millimeters.
Laboratory animal research fields require extremely high-precision dosage control. Specialized micro-syringes with only 0.1mL capacity achieve 0.01mL graduation precision, meeting precise dosing needs for mice, rats, and other laboratory animals. These products typically feature special fixation devices ensuring precise operation under animal anesthesia.
Military and police working dog diabetes treatment must consider battlefield or duty environment special characteristics. Specially developed portable kits contain pre-loaded medication syringes, alcohol swabs, and waste collection bags, enabling rapid treatment completion under field conditions. Packaging employs waterproof and shock-resistant designs adapting to harsh environmental conditions.
Large animals like horses require large-capacity syringes for insulin treatment. Specialized veterinary insulin syringes may hold 3-5mL capacity with thicker needles adapting to equine skin thickness. These products typically feature extension tubing for convenient injection while horses remain standing.
Training Certification Systems and Professional Development
To ensure proper veterinary insulin syringe usage, establishing comprehensive training certification systems proves crucial. This affects not only treatment outcomes but also animal safety and welfare.
Veterinary professional training typically divides into basic theory and practical operation components. Basic theory includes diabetes pathophysiology, insulin pharmacology, syringe technology principles, and safety protocols. Practical operation involves standardized procedural training ensuring accurate execution of every step.
Pet owner training emphasizes practicality and safety. Training content includes diabetes fundamentals, syringe usage methods, dosage calculation, injection techniques, and emergency situation handling. Through video instruction, physical demonstrations, and repeated practice, pet owners can independently and safely conduct home treatment.
Certification system establishment provides training quality assurance. Professional institutions can establish veterinary insulin syringe usage certification through theoretical examinations and practical assessments evaluating trainee mastery levels. Certified individuals possess professional skills while accepting corresponding professional responsibilities.
Continuing education mechanisms ensure practitioners stay current with new technologies and standards. With continuously advancing veterinary insulin syringe technology, regular training updates become necessary. Through online learning platforms, academic conferences, and professional journals, practitioners can continuously enhance professional capabilities.
International exchange and cooperation promote training standard unification and improvement. Through international veterinary organization coordination, different countries and regions can share best practice experiences, establishing unified training standards and certification systems. This international cooperation helps improve global veterinary insulin syringe usage levels.
Innovation Technology Prospects and Development Opportunities
Technological innovation in veterinary insulin syringes accelerates rapidly, with emerging technology applications bringing unprecedented development opportunities to the industry. These innovations will not only improve product performance but also redefine diabetic animal treatment experiences.
Nanotechnology applications bring new possibilities for veterinary insulin syringes. Nano-coating technology can create ultra-slick surfaces on needle surfaces, further reducing penetration resistance and pain. Nano-silver coatings also possess antimicrobial effects, reducing infection risks. These nanotechnology applications will elevate syringe performance to new heights.
3D printing technology provides new avenues for personalized customization. Based on specific animal anatomical characteristics, customized syringe accessories like special-angle needles or animal-conforming grips can be 3D printed. This personalized customization can further improve treatment precision and comfort.
Internet of Things (IoT) technology integration will achieve intelligent treatment management. Sensor-equipped syringes can automatically record injection times, dosages, and injection sites, with data transmitted via wireless networks to cloud platforms. Veterinarians and pet owners can monitor treatment situations in real-time through smartphone applications, enabling timely treatment adjustments.
Artificial intelligence technology shows enormous potential in dosage optimization. Machine learning algorithms analyzing extensive treatment data can create personalized insulin dosage protocols for individual animals. This AI-driven precision treatment will significantly improve treatment outcomes while reducing glucose fluctuations.
Biodegradable material applications will address environmental concerns. New bioplastic materials maintain medical-grade performance while rapidly degrading in natural environments. These eco-friendly material applications will greatly reduce medical waste environmental impact, aligning with sustainable development requirements.
The Mission and Value of Veterinary Insulin Syringes
Through this comprehensive analysis, we have gained deep insights into the crucial role and multifaceted value of veterinary insulin syringes in modern animal healthcare. These seemingly simple medical devices actually carry complex technical content and profound humanitarian care.
From a technological perspective, veterinary insulin syringes represent advanced levels of medical device manufacturing. Precision engineering design, strict quality control, innovative material applications, and user-friendly experience design combine perfectly to create high-performance medical products. With continuous technological advancement, we have every reason to believe future veterinary insulin syringes will become more intelligent, safe, environmentally friendly, and efficient.
From a medical value standpoint, veterinary insulin syringes provide reliable technical support for diabetic animal treatment. Standardized operational procedures, precise dosage control, excellent safety performance, and positive user experiences combine to make complex diabetes treatment simple and feasible, enabling more affected animals to receive timely and effective care.
From a social significance perspective, widespread veterinary insulin syringe applications reflect modern society’s respect and care for animal life. Under the guidance of harmonious human-animal coexistence principles, we willingly invest advanced technology and quality resources to provide optimal medical assurance for our animal companions. This care not only reflects human civilization progress but also promotes social harmony development.
From an economic angle, rapid veterinary insulin syringe market development creates tremendous economic value. This vibrant market provides development opportunities for manufacturers while creating employment opportunities for related service industries. Simultaneously, high-quality medical devices improve treatment efficiency, reduce overall medical costs, and create positive economic benefits.
Looking forward, the veterinary insulin syringe industry faces unprecedented development opportunities. Accelerating technological innovation, sustained market demand growth, continuously improving regulatory environments, and increasingly close international cooperation converge to provide powerful momentum for industry development. We are confident that through collective efforts from all parties, veterinary insulin syringes will play increasingly important roles in animal health protection endeavors.
Ultimately, the value of veterinary insulin syringes lies not only in their technological sophistication and economic benefits but also in the life care and social responsibility they embody. Behind every syringe stands an animal needing help, an expectant family, and a precious life. This profound significance elevates veterinary insulin syringes beyond mere medical device categories, making them important bridges connecting technology with compassion, professionalism with responsibility, and innovation with tradition.
This content is for reference purposes only. Specific treatment protocols should be developed in consultation with professional veterinarians. Animal health requires professional medical guidance—never attempt self-diagnosis or treatment.