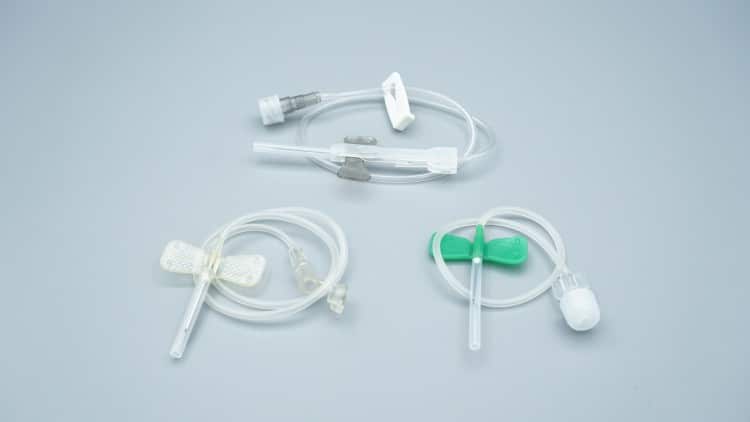
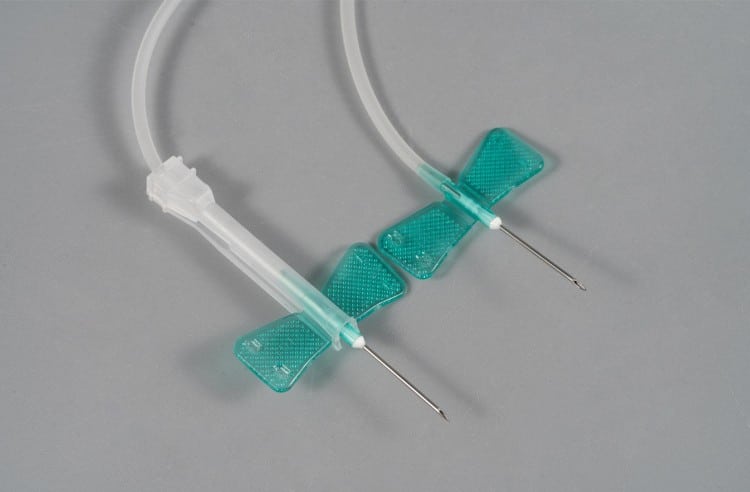
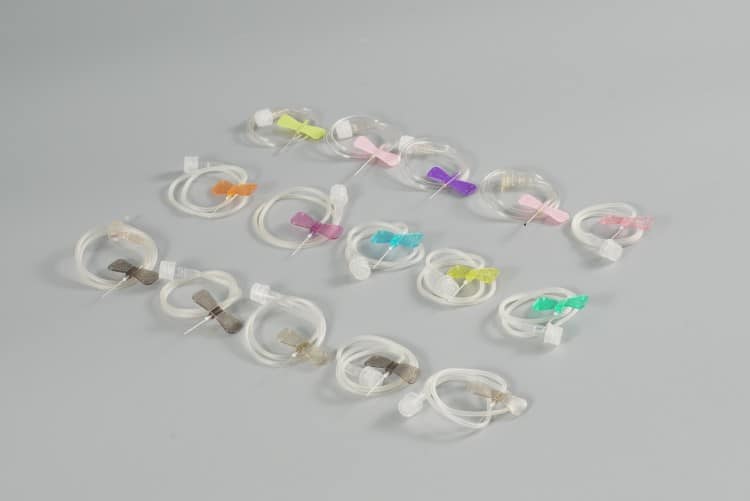
Butterfly needles (Winged Infusion Sets) are precision-engineered, disposable medical devices that we custom manufacture for global healthcare clients. As a leading medical device manufacturer, we specialize in providing high-quality, customized butterfly needle solutions for distributors, hospitals, and healthcare systems worldwide, particularly targeting the European and North American markets.
Product Architecture & Engineering Excellence
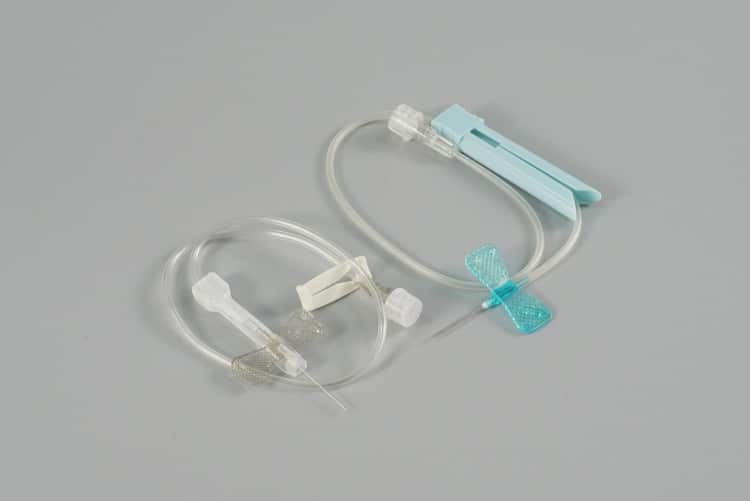
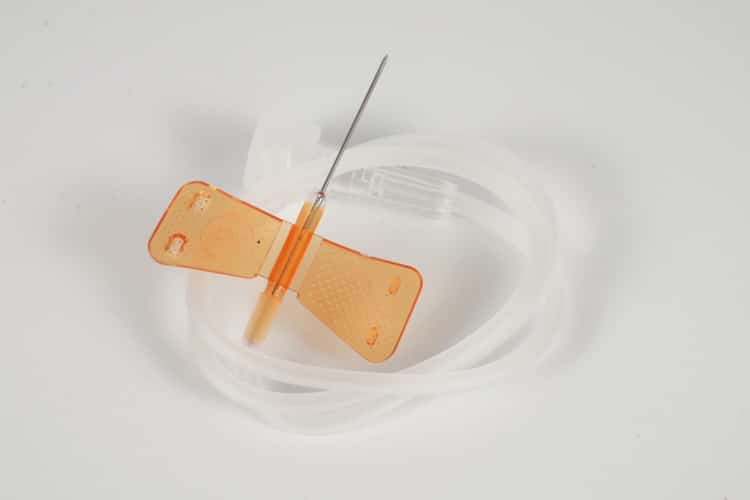
Core Components
- Needle Hub: Premium stainless steel construction, available in 18G to 27G specifications, ensuring optimal sharpness retention and biocompatibility
- Butterfly Wings: Ergonomically designed flexible plastic wings for superior grip stability and secure patient anchoring
- Transparent Tubing: Medical-grade PVC tubing (15-30cm options) enabling real-time blood flashback visualization
- Connector Interface: Universal Luer-compatible connections ensuring seamless integration with infusion systems
Custom Manufacturing Services
- Specification Customization: Tailored needle gauges and tubing lengths per client requirements
- Private Labeling: Personalized packaging design and branding solutions
- Regulatory Compliance: Meeting FDA 510(k), CE Mark, and ISO 13485 standards globally
- Scalable Production: Flexible manufacturing capacity for high-volume orders
Design Advantages
- Triple-bevel Technology: Minimizes insertion trauma and maximizes first-stick success rates
- Silicone Coating: Ultra-smooth needle surface reduces tissue damage and patient discomfort
- Crystal-clear Visualization: Immediate blood return confirmation for procedural accuracy
- Anti-dislodgement Design: Wings provide secure fixation preventing accidental removal
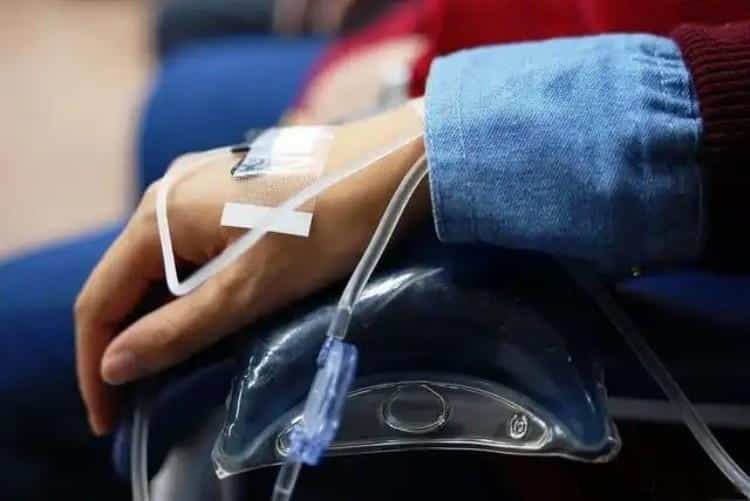
Technical Specifications & Performance Metrics
| Gauge | Needle Length | Tubing Length | Primary Applications | Flow Rate |
|---|---|---|---|---|
| 27G | 13mm | 15cm | Neonatal/Pediatric | 0.5 mL/min |
| 25G | 16mm | 20cm | Geriatric/Pediatric | 1.2 mL/min |
| 23G | 19mm | 25cm | Adult Standard | 2.8 mL/min |
| 21G | 25mm | 30cm | Rapid Infusion | 4.5 mL/min |
Critical Clinical Applications
ESSENTIAL Use Cases
1. Pediatric Healthcare
- NICU (Neonatal Intensive Care): Ultra-fine needles for fragile vascular access
- Pediatric Oncology: Chemotherapy delivery with precision dosing
- Immunization Programs: Reduced anxiety and improved compliance
2. Geriatric Medicine
- Fragile Vessel Management: Ideal for elderly patients with compromised vasculature
- Long-term Care Facilities: Minimized vessel trauma during repeated access
- Palliative Care: Comfort-focused medication delivery
3. Emergency Medicine
- Trauma Response: Rapid vascular access in critical situations
- Code Blue Protocols: Reliable drug delivery during resuscitation
- Blood Sampling: Multiple draws with single puncture
4. Oncology Services
- Chemotherapy Infusion: Controlled drug delivery with enhanced safety
- Blood Transfusions: Optimal flow rates for cellular products
- Supportive Care: Nutrition and hydration therapy
5. Outpatient Settings
- Same-day Surgery: Short-term access for procedural needs
- Imaging Centers: Contrast media injection with precision control
- Diagnostic Labs: High-quality specimens with minimal hemolysis
Competitive Analysis – Market Leadership
vs. Traditional Straight Needles
| Performance Factor | Butterfly Needles | Straight Needles |
|---|---|---|
| Procedural Success Rate | 95-98% | 85-90% |
| Patient Comfort Score | ★★★★★ | ★★★ |
| Operator Confidence | ★★★★★ | ★★★ |
| Stability Rating | ★★★★★ | ★★ |
| Cost Efficiency | ★★★ | ★★★★★ |
vs. IV Catheters
| Application | Butterfly Needles | IV Catheters |
|---|---|---|
| Short-term Use (<4 hours) | OPTIMAL | Standard |
| Patient Mobility | Limited | SUPERIOR |
| Infection Risk | LOWER | Higher |
| Ease of Insertion | SUPERIOR | Standard |
| Cost per Procedure | LOWER | Higher |
vs. Scalp Vein Sets
| Clinical Factor | Butterfly Needles | Scalp Vein Sets |
|---|---|---|
| Pediatric Suitability | ★★★★★ | ★★★★ |
| Fixation Security | ★★★★★ | ★★★ |
| Visual Confirmation | ★★★★★ | ★★★★ |
| Professional Preference | ★★★★★ | ★★★ |
Quality Assurance & Regulatory Excellence
Manufacturing Standards
- ISO 13485 Medical Device Quality Management System
- FDA 510(k) clearance for US market distribution
- CE Marking compliance for European market access
- cGMP (Current Good Manufacturing Practices) adherence
- 100% quality inspection on every production batch
Safety Certifications
- Single-use Sterile: ETO sterilization ensuring zero contamination risk
- Biocompatibility Testing: USP Class VI material validation
- Needle Safety Features: Integrated safety mechanisms preventing needlestick injuries
- Latex-free Construction: Allergen-free for sensitive patients
Global Market Positioning
Target Markets
- North America: Focus on hospital systems, GPOs, and healthcare distributors
- European Union: Compliance with MDR regulations and regional quality standards
- Emerging Markets: Cost-effective solutions for expanding healthcare infrastructure
Market Advantages
- Competitive Pricing: 20-30% cost savings compared to premium brands
- Reliable Supply Chain: Global distribution network with consistent availability
- Technical Support: Multilingual customer service and clinical education programs
- Customization Capability: Tailored solutions for specific market requirements
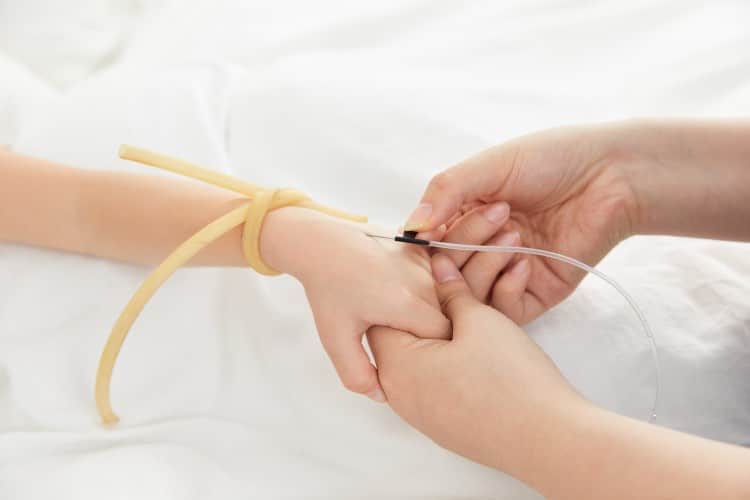
Clinical Best Practices & Usage Guidelines
Optimal Technique
- Gauge Selection: Match needle size to patient demographics and clinical requirements
- Proper Handling: Butterfly wing grip ensures maximum control and stability
- Insertion Angle: 15-30 degree approach prevents vessel perforation
- Flashback Confirmation: Visual blood return confirms successful venipuncture
- Secure Fixation: Tape anchoring prevents accidental dislodgement
Safety Protocols
- Strict aseptic technique preventing healthcare-associated infections
- Single-use policy eliminating cross-contamination risks
- Proper disposal in sharps containers per OSHA guidelines
- Staff training programs ensuring optimal clinical outcomes
Innovation & Future Development
Current Technologies
- Safety-engineered Needles: Retractable mechanisms preventing occupational exposure
- Enhanced Visualization: Improved tubing clarity for better blood return detection
- Ergonomic Improvements: Advanced wing design for superior handling
- Smart Materials: Temperature-sensitive indicators for enhanced safety
Emerging Innovations
- Digital Integration: RFID tracking for inventory management
- Biodegradable Materials: Eco-friendly alternatives for sustainable healthcare
- Nanotechnology Coatings: Ultra-low friction surfaces for painless insertion
- AI-assisted Design: Data-driven optimization for patient-specific solutions
Partnership Opportunities
For Distributors
- Exclusive territory rights in key geographic markets
- Marketing support including clinical education materials
- Competitive margin structures enabling profitable growth
- Just-in-time delivery reducing inventory carrying costs
For Healthcare Systems
- Volume pricing agreements for large-scale procurement
- Clinical trials support demonstrating outcome improvements
- Staff training programs ensuring optimal product utilization
- Customized packaging for specific workflow requirements
Conclusion
As a premier medical device manufacturer, we deliver world-class butterfly needle solutions that meet the demanding requirements of global healthcare markets. Our commitment to quality excellence, regulatory compliance, and customer satisfaction positions us as the preferred manufacturing partner for distributors and healthcare organizations worldwide.
Through advanced manufacturing capabilities, stringent quality controls, and responsive customer service, we enable our clients to provide superior patient care while achieving optimal business outcomes. Partner with us to access industry-leading butterfly needle technology that sets new standards for clinical excellence and patient safety.
Key Differentiators:
- ✅ FDA & CE Certified Manufacturing
- ✅ Custom OEM/ODM Solutions
- ✅ Competitive Global Pricing
- ✅ Reliable Supply Chain
- ✅ 24/7 Technical Support
This guide is provided by a professional medical device manufacturer for healthcare professionals and industry partners. All products must be used in accordance with applicable medical standards and product specifications.