The vacuum blood collection system, first introduced by Becton Dickinson in 1947, has evolved into the gold standard for modern clinical laboratory blood sample collection. With over 14 billion blood tests performed annually worldwide (WHO data), approximately 85% utilize vacuum collection systems. For healthcare procurement professionals, distributors, and laboratory administrators, selecting the right blood collection tubes directly impacts not only diagnostic accuracy but also operational efficiency and cost-effectiveness.
Fundamental Principles and Classification Systems
Operational Mechanism
Vacuum blood collection tubes utilize pre-established negative pressure to automatically draw predetermined blood volumes through double-ended needles. This standardized collection method significantly reduces human error while ensuring consistent sample quality – a critical factor for laboratory accreditation and regulatory compliance.
International Classification Standards
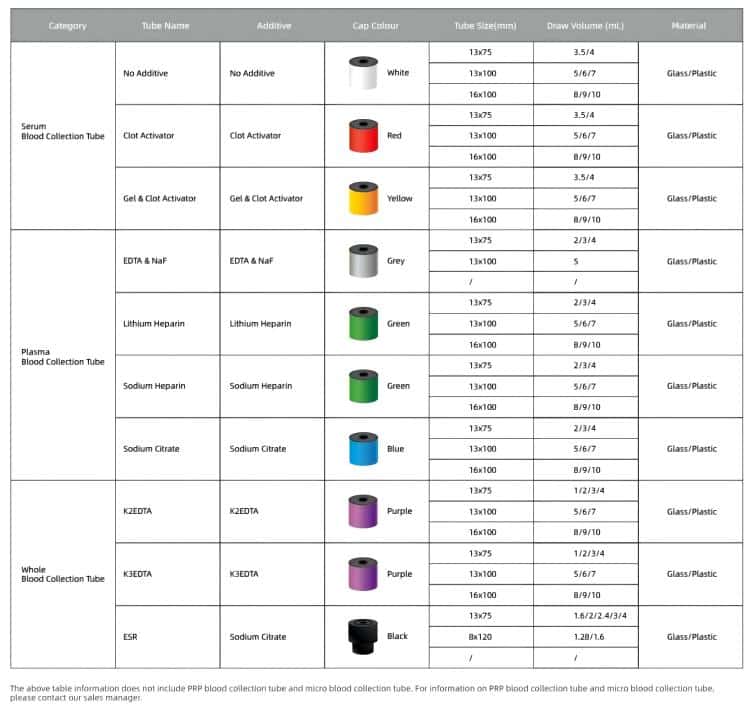
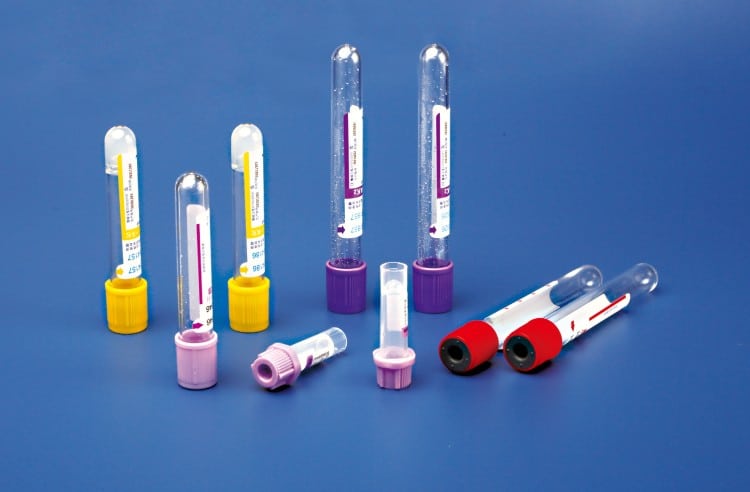
According to ISO 6710 specifications, vacuum blood collection tubes are primarily identified through standardized cap color coding systems:
Red Top Tubes (No Additives/Serum)
- Applications: Clinical chemistry, immunoassays
- Clotting time: 30-60 minutes
- Cost efficiency: Ideal for high-volume chemistry panels
- Suitable tests: Liver function, renal function, lipid panels, protein analysis
Purple Top Tubes (EDTA Anticoagulant)
- Applications: Hematology testing
- Anticoagulant: Ethylenediaminetetraacetic acid (EDTA)
- Market demand: Highest volume due to routine CBC testing
- Suitable tests: Complete blood count, blood typing, HbA1c
Blue Top Tubes (Sodium Citrate Anticoagulant)
- Applications: Coagulation studies
- Blood-to-anticoagulant ratio: 9:1
- Specialized requirements: Critical for accurate PT/INR monitoring
- Suitable tests: PT, APTT, D-dimer, factor assays
Green Top Tubes (Heparin Anticoagulant)
- Applications: Plasma chemistry
- Anticoagulant options: Lithium heparin or sodium heparin
- Fast turnaround: No clotting time required
- Suitable tests: Blood gas analysis, electrolytes, STAT chemistry
Strategic Selection Criteria for Procurement Professionals
1. Test Menu Optimization and Cost Management
Clinical Chemistry Portfolio Strategy For routine biochemistry testing, serum samples consistently outperform plasma samples in analytical accuracy. The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) research demonstrates that serum samples reduce matrix effects by 15-20%, translating to fewer repeat tests and improved cost-per-result ratios. High-volume laboratories should prioritize red top or gold top tubes (with separator gel) for liver function, renal function, and lipid testing to maximize operational efficiency.
Hematology Volume Considerations Complete blood count testing requires EDTA anticoagulant tubes exclusively. The Clinical and Laboratory Standards Institute (CLSI) H3-A6 guidelines specify EDTA concentrations between 1.5-2.2mg/mL. For bulk purchasing, ensure supplier quality control maintains these specifications – concentration variations can lead to cell morphology artifacts and counting errors, potentially requiring costly retesting.
2. Supply Chain Reliability and Sample Stability
Time-Critical Testing Requirements Different assays exhibit varying time sensitivity profiles that directly impact laboratory workflow and staffing requirements. Blood gas analysis samples must be processed within 30 minutes, while certain biochemistry markers like total protein and albumin remain stable for 24 hours at room temperature. The International Federation of Clinical Chemistry (IFCC) data shows that processing delays result in glucose degradation of 5-7% per hour and potassium elevation of 0.1-0.2mmol/L hourly – factors that procurement teams must consider when evaluating supplier delivery reliability.
Cold Chain Management Coagulation testing samples are extremely temperature-sensitive, requiring specialized handling protocols. The International Society on Thrombosis and Haemostasis (ISTH) mandates that sodium citrate anticoagulated plasma samples be stored at 2-8°C, with coagulation factor activity declining significantly after 4 hours. This requirement necessitates robust cold chain logistics and may influence supplier selection criteria.
3. Additive Compatibility and Quality Assurance
Anticoagulant Selection: Scientific and Economic Rationale EDTA functions by chelating calcium ions to prevent coagulation but may affect certain enzyme activities, potentially impacting specialized assays. Heparin activates antithrombin III but can interfere with immunoassays, requiring careful test menu planning. Sodium citrate provides reversible calcium binding, essential for coagulation testing where calcium can be re-added during analysis. Understanding these mechanisms helps procurement professionals make informed decisions about tube inventory optimization.
Cross-Contamination Prevention Protocols WHO quality management guidelines emphasize that collection sequence significantly impacts result accuracy. The recommended draw order – blood culture → sodium citrate → serum → heparin → EDTA → sodium fluoride – prevents additive cross-contamination. This standardized approach reduces re-collection costs and improves patient satisfaction scores.

Global Regulatory Compliance and Standardization
International Standards Evolution
ISO 6710 standards have undergone multiple revisions since 1995, with the 2017 edition emphasizing international cap color uniformity and precise additive concentration control. Global laboratory management systems now align with these standards, creating opportunities for standardized procurement across multiple markets.
Regional Adaptations and Market Considerations
Despite high standardization levels, regional variations persist. Japanese JIS T 3225 standards impose stricter technical requirements than ISO specifications, while EU EN 14820 standards prioritize environmental sustainability. These variations create both challenges and opportunities for suppliers serving international markets.
Manufacturing Quality Control Systems
The FDA and European Medicines Agency (EMA) require tube manufacturers to maintain rigorous quality management systems. Leading global manufacturers like BD, Greiner Bio-One, and Sarstedt achieve quality control precision of ±2%, providing procurement professionals with reliable performance benchmarks for supplier evaluation.
Common Procurement Pitfalls and Risk Mitigation
Pitfall 1: “Universal Tube” Misconception
Many procurement decisions are based on the incorrect assumption that certain tubes can serve all testing applications. Different assays require specific sample matrices, and inappropriate tube selection can cause result deviations of 20-30%, leading to increased healthcare costs and potential patient safety issues.
Pitfall 2: Fill Volume Specification Oversight
Tube fill volume directly affects blood-to-additive ratios, with CLSI standards requiring 90% of nominal capacity. Underfilled tubes create excessive anticoagulant concentrations, compromising test accuracy and potentially necessitating costly repeat collections.
Pitfall 3: Standardization of Mixing Protocols
Different additives require specific mixing procedures: EDTA tubes need gentle 8-10 inversions, sodium citrate tubes require 3-4 inversions, while serum tubes need minimal agitation. Improper mixing can cause hemolysis, affecting test quality and laboratory efficiency.
Quality Control and Performance Evaluation Framework
Pre-Analytical Quality Management
Research indicates that 60-70% of laboratory errors originate in the pre-analytical phase, with incorrect tube selection accounting for 15-20% of these errors. Implementing comprehensive pre-analytical quality control systems – including tube selection verification, fill volume monitoring, and standardized mixing protocols – can reduce error rates significantly, improving both patient outcomes and laboratory economics.
Performance Evaluation Metrics
Key performance indicators for tube evaluation include: vacuum stability (±10%), additive homogeneity (coefficient of variation ≤5%), material compatibility (hemolysis rate ≤0.5%), and sealing integrity (leak rate ≤0.1%). These metrics provide objective criteria for supplier performance assessment and contract negotiations.
Cost-Benefit Analysis Framework
Total cost of ownership extends beyond unit price to include storage requirements, shelf life, training costs, and potential re-collection expenses. Leading procurement organizations utilize comprehensive cost models that factor in these variables to optimize supplier selection and inventory management strategies.
Market Trends and Future Innovations
Technology Integration Opportunities
Next-generation vacuum collection tubes incorporate smart technologies including RFID tagging for automated sample tracking, pre-separation technology reducing turnaround times by 30-40%, and integrated barcode systems for seamless laboratory information system integration. These innovations offer significant operational advantages for high-volume laboratories.
Sustainability and Environmental Considerations
Environmental consciousness drives adoption of biodegradable materials and recyclable designs in tube manufacturing. Bio-based plastics and sustainable packaging solutions are becoming key differentiators in supplier selection, particularly for European markets where environmental regulations are stringent.
Customization and Private Label Opportunities
The growing trend toward laboratory consolidation creates opportunities for customized tube solutions and private label manufacturing. Volume buyers can leverage these trends to negotiate better pricing and tailored product specifications that align with their specific operational requirements.
Supplier Selection and Partnership Development
Manufacturing Capability Assessment
Evaluate suppliers based on production capacity, quality certifications (ISO 13485, FDA 510(k)), regulatory compliance history, and technical support capabilities. Consider geographic distribution networks and regional manufacturing presence to optimize supply chain resilience.
Innovation Partnership Potential
Forward-thinking suppliers invest in R&D collaborations with laboratory customers to develop next-generation solutions. These partnerships can provide early access to innovative technologies and customized solutions that deliver competitive advantages.
Risk Management Considerations
Develop supplier diversification strategies to mitigate supply chain risks. Consider factors such as raw material sourcing, manufacturing location diversity, and alternative supplier qualification to ensure continuity of operations during market disruptions.
Conclusion
Strategic selection of vacuum blood collection tubes represents a critical procurement decision that impacts laboratory efficiency, diagnostic accuracy, and operational costs. Healthcare procurement professionals must balance multiple factors including test menu requirements, quality specifications, regulatory compliance, and total cost of ownership to optimize their selection process.
The evolution toward more sophisticated, technology-integrated solutions creates both opportunities and challenges for procurement teams. Success requires deep understanding of clinical requirements, market dynamics, and supplier capabilities, combined with strategic partnership development and continuous performance monitoring.
By implementing comprehensive evaluation frameworks and maintaining focus on quality, compliance, and innovation, procurement professionals can ensure their organizations have access to the most appropriate vacuum collection tube solutions for their specific operational requirements.
About Our Manufacturing Excellence
With 15 years of specialized experience in vacuum blood collection tube manufacturing, we have established ourselves as a trusted partner for healthcare distributors and laboratory networks across Europe and North America. Our state-of-the-art facilities maintain ISO 13485 certification and FDA compliance, ensuring consistent quality that meets the most stringent international standards.
Our commitment to innovation, quality, and customer partnership has made us the preferred supplier for organizations seeking reliable, high-performance vacuum collection tube solutions. Whether you require standard configurations or customized specifications, our experienced team delivers the expertise and manufacturing excellence that today’s healthcare market demands.
Contact us to discover how our manufacturing capabilities can support your laboratory’s success and your patients’ care quality.