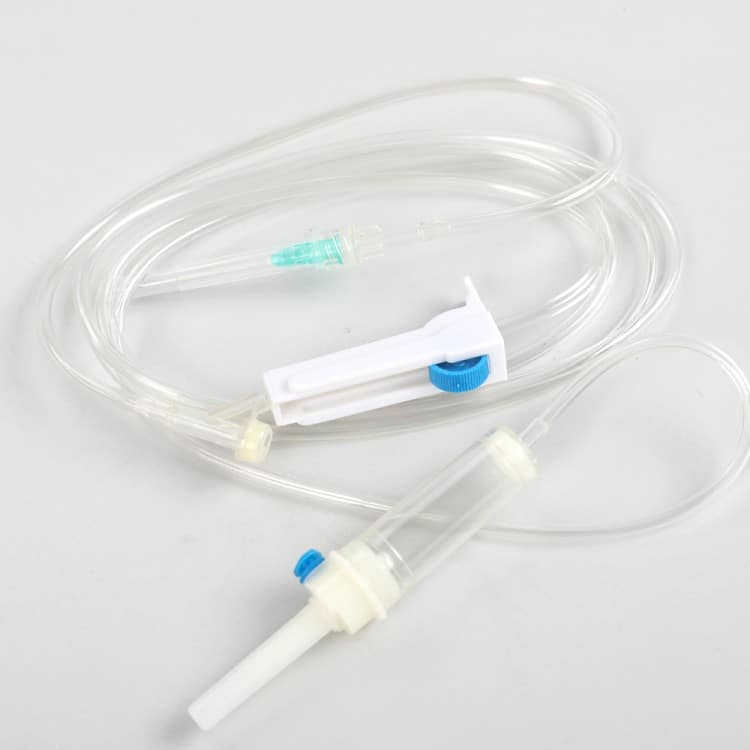
In clinical treatment, medical safety infusion sets are critical tools for ensuring safe intravenous drug administration. Selecting the right product not only effectively prevents needlestick injuries and infusion accidents but also improves treatment efficiency and ensures patient comfort. As a professional factory with years of experience in medical device manufacturing, we aim to provide hospital procurement managers and general users with a comprehensive, practical purchasing guide.
Why Is Selecting the Right Infusion Set Crucial?
According to the Chinese Medical Device Industry Association, over 65% of infusion-related adverse events are associated with improper product selection or non-standard usage of infusion sets. This not only increases medical risks but also causes cost waste and nursing pressure. Therefore, selecting reliable products from the source is the first step to improving treatment safety.
Types of Medical Safety Infusion Sets
We categorize infusion sets by different applications and performance requirements:
1. Classification by Safety Protection Function
Active Safety Infusion Sets
- Automatic needle retraction with virtually no needlestick risk
- User-friendly operation, suitable for high-intensity departments like emergency rooms, ICUs, and hematology
- Recommended for high-risk environments
Passive Safety Infusion Sets
- Manual activation of safety device, requires appropriate training before use
- More cost-effective, suitable for routine use scenarios such as general wards and outpatient clinics
2. Classification by Flow Accuracy
Precision Infusion Sets
- More precise drip rate control with error within ±5%
- Suitable for pediatrics, cardiology, critical care, and other departments sensitive to flow rates
Standard Infusion Sets
- Drip rate control accuracy meets most clinical requirements
- Cost-effective, the main model for routine procurement in major hospitals
3. Classification by Material
PVC Material (High market share, good cost-effectiveness)
- Transparent tubing with high strength
- Complies with international biocompatibility standards, suitable for most adult patients
Non-PVC Materials (such as TPE, PE)
- DEHP-free, environmentally safe
- Particularly suitable for children, pregnant women, and other populations sensitive to plasticizers
Department-Specific Selection Guidelines
Based on actual procurement experience from multiple hospitals, we summarize the following recommended configurations:
ICU Critical Care Units
- Active safety type + precision drip control + multi-channel design
- High-intensity use environments require greater durability and stability
- Recommend high-performance needlestick prevention and antimicrobial coating features
Pediatric Wards
- Precision drip control + non-PVC materials + color-coded identification
- Children have higher requirements for flow accuracy and material safety
- Recommend products specifically designed for small-volume infusions
General Internal Medicine
- Passive safety type + PVC materials + good compatibility
- Suitable for high-volume, fast-turnover departments
- Better cost-effectiveness, recommend bulk purchasing
Oncology Departments
- Active safety type + chemotherapy-compatible materials + light-resistant tubing
- Chemotherapy drugs are highly corrosive, requiring high product compatibility and sealing
- Anti-static and long-term infusion designs are particularly important
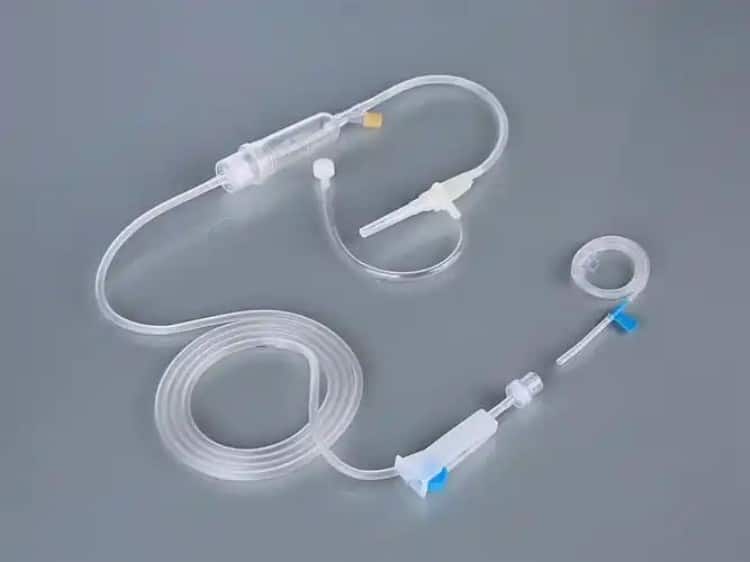
Key Performance Indicators: Simple Assessment Criteria
We recommend checking the following key performance indicators before procurement:
| Performance Category | Key Indicators | Recommended Standards |
|---|---|---|
| Safety | Needlestick protection success rate | ≥99.5% |
| Accuracy | Drip rate error range | ±5% or less (precision type) |
| Sealing | Connection joint strength | ≥5kgf, no leakage |
| Biocompatibility | No cytotoxicity, hemolysis rate <5% | Complies with ISO 10993/USP standards |
All our products have passed ISO 13485 Quality Management System Certification and possess National Medical Device Registration Certificates and CE Certification (for export).
Cost-Benefit Analysis: Return on Investment
Example: 500-bed hospital
- Annual additional investment for upgrading to safety infusion sets: approximately $22,000
- Annual indirect cost savings from reducing needlestick injuries and infusion accidents: approximately $37,000
- Return on Investment (ROI): 68.2%
This doesn’t include soft benefits such as improved healthcare worker satisfaction and optimized nursing efficiency from reducing adverse events.
How to Select Qualified Suppliers
As a professional manufacturer, we recommend focusing on the following key points:
Basic Qualifications
- Medical device manufacturing license
- ISO 13485 quality certification
- Complete national registration certificates and CE certificates
Production Capacity and Service Capability
- Monthly production capacity ≥500,000 units, sufficient inventory
- Comprehensive training and technical support
- After-sales response within 24 hours, major issues resolved within 48 hours
Risk Control and Supply Assurance
- Multiple raw material sources and comprehensive emergency mechanisms
- Full product liability insurance coverage to reduce procurement risks
We support OEM customization services and provide flexible combinations of various sizes, functions, and packaging formats to meet the customization needs of different hospitals and distributors.
Daily Use Management Recommendations
To ensure the safe use of every infusion set, we recommend hospitals and clinics implement the following procedures:
Pre-use 5-Step Inspection
- Check package integrity
- Verify product specifications
- Inspect tubing and connections for damage
- Confirm safety device functionality
- Verify patient information
During-use Monitoring
- Check drip rate, puncture site, and air bubbles every 15-30 minutes
- Replace immediately if problems occur
Post-use Handling
- Activate needle protection device
- Properly segregate medical waste
- Record any abnormalities for improvement
Professional Standards Through Focus: Choose Trusted Manufacturers
As a manufacturing facility with over 15 years of experience in medical consumables production, we consistently maintain our product philosophy of safety, stability, and high cost-effectiveness, committed to providing high-quality solutions for medical institutions.
Procurement Recommendations Summary:
Key Selection Criteria:
- High-risk areas: Choose active safety types
- Pediatric use: Prioritize non-PVC and high-precision options
- General departments: Focus on cost-effectiveness and compatibility
- Supplier selection: Partner with qualified, stable, and reliable manufacturers
- Quality management: Emphasize user training and comprehensive quality control
Critical Performance Standards:
Safety Requirements:
- Needlestick injury prevention rate: >99%
- Activation force: 5-15N (Newtons)
- Complete needle retraction time: <0.5 seconds
Flow Control Specifications:
- Precision sets: 150±7 drops/mL drop factor
- Standard sets: 20±1 drops/mL drop factor
- Flow accuracy: ±5% (precision), ±10% (standard)
Material Standards:
- PVC transparency: ≥90%
- Tensile strength: ≥15MPa
- Bacterial endotoxin limit: <0.5EU/mL
- Hemolysis rate: <5%
Market Positioning and Costs:
| Product Type | Unit Price Range | Annual Consumption | Annual Cost |
|---|---|---|---|
| Standard Type | $0.85-1.40 | 30,000 units | $25,500-42,000 |
| Safety Type | $1.70-2.85 | 25,000 units | $42,500-71,250 |
| Precision Type | $2.10-3.55 | 15,000 units | $31,500-53,250 |
We welcome you to contact us anytime for sample testing, technical consultation, or customization services. Let us work together to improve clinical safety and protect every patient’s health.
If you are a hospital procurement manager, distributor, or clinic administrator, please contact us for detailed product specifications or factory visit materials. We look forward to being your long-term reliable partner.
Contact Us | Learn More | Get Quote
This guide is based on industry standards, clinical practice surveys, and relevant literature. Please consult technical experts when necessary for specific selection decisions.